12.5: Vitamin K
- Page ID
- 40997
There are 3 forms of vitamin K. Phylloquinone (K1), the plant form of vitamin K, is the primary dietary form of vitamin K. Its structure is shown below.

Green leafy vegetables, broccoli, Brussels sprouts, and asparagus are foods that are good sources of phylloquinone2.
Another form of vitamin K, menaquinone (K2), is synthesized by bacteria in the colon (and potentially elsewhere). Menaquinone comprises ~10% of absorbed vitamin K every day and can also be found in small amounts in animal products. Its structure is shown below3.
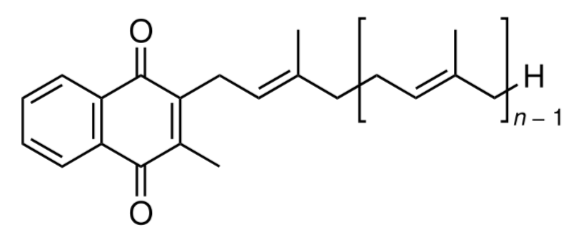
In the structure above, if it was menaquinone-8, there would be 7 (8-1) repeating units of the structure inside the brackets above.
The synthetic form of vitamin K is menadione (K3), whose structure is shown below.

A tail, similar to the one found in menaquinone, has to be added to menadione for it to be biologically active.
Vitamin K is absorbed like other fat-soluble substances. Approximately 80% of phylloquinone and menaquinone are incorporated into chylomicrons and stored primarily in the liver2,6. Once metabolized, vitamin K is primarily excreted via bile in the feces, with a lesser amount excreted in urine6.
Query \(\PageIndex{1}\)
Vitamin K Functions
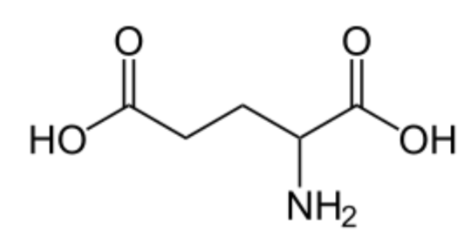
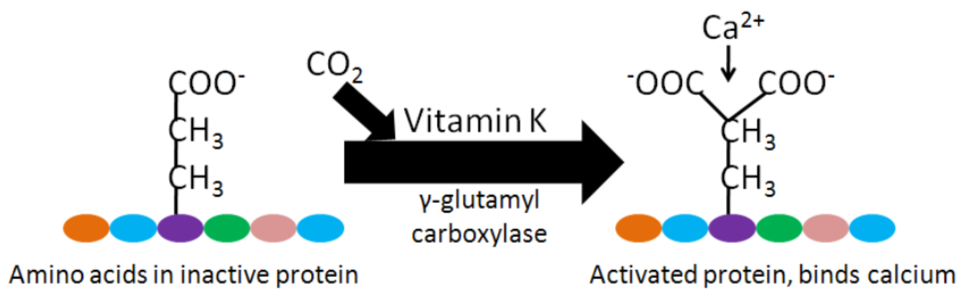
Vitamin K is a cofactor for carboxylation reactions that add \(\ce{CO2}\) to the amino acid, glutamic acid (glutamate), in certain proteins. The structure of glutamic acid is shown below.
The enzyme, gamma-glutamyl carboxylase, uses a vitamin K cofactor to convert glutamic acid to gamma-carboxyglutamic acid (Gla). In the process of serving as a cofactor, vitamin K is converted to vitamin K epoxide (structure in the figure below).
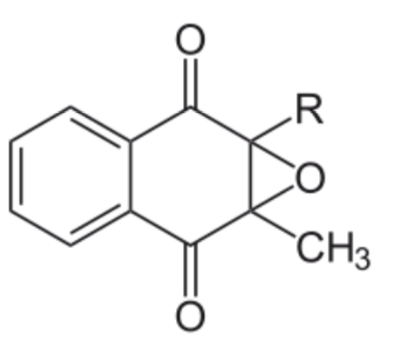
Vitamin K epoxide needs to be converted back to vitamin K to serve as a cofactor again. Gla proteins are those that contain gamma-carboxyglutamic acid(s). This formation of gamma-carboxyglutamic acid allows the 2 positive charges of calcium to bind between the 2 negative charges on the carboxylic acid groups (\(\ce{COO-}\)) in the Gla. The binding of calcium activates these proteins2,3,6.
After being used as a cofactor by gamma-glutamyl carboxylase to produce a Gla protein, vitamin K becomes vitamin K epoxide. Vitamin K epoxide needs to be converted back to vitamin K to serve as a cofactor again. Activated Gla proteins are important in blood clotting. Blood clotting occurs through a cascade of events, as shown in the following video. The first video gives a detailed overview of this, the second video is a fun depiction of the blood clotting cascade.
Within the blood clotting cascade, there are a number of potential Gla proteins, as shown in the figure below.

If these proteins within the blood clotting cascade are not activated Gla proteins, the cascade does not proceed as normal, leading to impaired blood clotting. Warfarin (Coumadin) and dicumarol are a couple of blood thinning drugs that inhibit this regeneration of vitamin K. This reduces the amount of activated Gla proteins in the blood clotting cascade, thus reducing the clotting response10. The structure of warfarin and dicumarol are shown below.


The following coumadin rap song video gives further information on warfarin.
Vitamin K may also be important for bone health. There are 3 Gla proteins found in bone: osteocalcin, matrix Gla protein (MGP), and protein S10. Osteocalcin is a major bone protein, constituting 15-20% of all non-collagen proteins in bone. However, overall, the function of these 3 proteins in bone is not known3,6. Some research suggests that higher vitamin K status or intake decreases bone loss, but it is still not clear how important vitamin K is for bone health13.
Query \(\PageIndex{2}\)
Query \(\PageIndex{3}\)
Query \(\PageIndex{4}\)
Vitamin K Deficiency & Toxicity
Vitamin K deficiency is rare, but can occur in newborn infants. They are at higher risk, because there is poor transfer of vitamin K across the placental barrier (from mother to fetus in utero), their gastrointestinal tracts do not contain vitamin K producing bacteria, and breast milk is generally low in vitamin K (most infant formula is fortified)6. As a result, it is recommended (and widely practiced) that all infants receive a vitamin K injection within 6 hours of birth3.
Prolonged antibiotic treatment (which kills bacteria in the gastrointestinal tract, including those that produce menaquinone) and lipid absorption problems can also lead to vitamin K deficiency2. Vitamin K deficient individuals have an increased risk of bleeding or hemorrhage. Recall that high levels of alpha-tocopherol intake (levels that generally would only be achieved through supplementation) can also interfere with vitamin K's blood clotting function. It is believed that an alpha-tocopherol metabolite, with similar structure to the vitamin K forms you learned about, antagonizes the action of vitamin K.
Phylloquinone and menaquinone have no reported toxicities. However, menadione can cause liver damage6.
Query \(\PageIndex{5}\)
Query \(\PageIndex{6}\)
References
- https://en.Wikipedia.org/wiki/Vitamin_K#/media/File:Phylloquinone_structure.svg
- McGuire M, Beerman KA. (2011) Nutritional sciences: From fundamentals to food. Belmont, CA: Wadsworth Cengage Learning.
- Byrd-Bredbenner C, Moe G, Beshgetoor D, Berning J. (2009) Wardlaw's perspectives in nutrition. New York, NY: McGraw-Hill.
- en.Wikipedia.org/wiki/File:Menaquinone.svg
- en.Wikipedia.org/wiki/File:Menadione.png
- Gropper SS, Smith JL, Groff JL. (2008) Advanced nutrition and human metabolism. Belmont, CA: Wadsworth Publishing.
- https://www.chemsynthesis.com/base/chemical-structure-423.html
- https://upload.wikimedia.org/Wikipedia/commons/1/18/Vitamin-K-Epoxid.svg
- https://s3-us-west-2.amazonaws.com/c...BDD65D5C1F.png
- Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, editors. (2006) Modern nutrition in health and disease. Baltimore, MD: Lippincott Williams & Wilkins.
- en.Wikipedia.org/wiki/File:Warfarin.svg
- en.Wikipedia.org/wiki/File:Dicumarol.svg
- Shea MK, Booth S. (2008) Update on the role of vitamin K in skeletal health. Nutr Rev 66(10): 549-557.


