9.3: Calcium- Critical for Bones and Throughout the Body
- Page ID
- 39997
As discussed on the previous page, bones are made up of two components: inorganic minerals and a protein matrix. Minerals, which make up 65% of bone tissue, are what gives bones their hardness. Calcium and phosphorus together form hydroxyapatite crystals, the main mineral component of bone. Other minerals, including magnesium, fluoride, sodium, and potassium, play supporting roles. This page focuses on calcium, and we’ll cover potassium, magnesium, and fluoride on the following page. (Sodium and potassium are covered in the electrolyte section of this text.)
Calcium Functions and Regulation
Calcium is the most abundant mineral in the body. Most of the body’s calcium—more than 99% of it—is stored in bone, where it’s important for bone strength and structure. The remaining 1% of the body’s calcium is found in the blood and soft tissues, but it is here that calcium performs its most critical functions. For example, calcium is required for the transmission of every nerve impulse, electrical signals sent from one nerve cell to another. It’s also required for every cycle of muscle contraction and relaxation. With inadequate calcium, muscles can’t relax, and instead become stiff and contract involuntarily, a condition known as tetany. Calcium also plays vital roles in blood pressure regulation, blood clotting, enzyme activation, hormone secretion, and signaling between cells.1
The many roles of calcium around the body are critical to daily survival, so maintaining homeostasis, or a steady state, of blood calcium levels is a high priority. The body rigorously controls blood calcium levels in a very tight range. If blood calcium drops, your body initiates several mechanisms to restore homeostasis, including drawing calcium from the bone. While the calcium stored in bone is important for long-term strength and structure of bone, it also serves as a calcium reserve that can be drawn upon to support the vital functions of calcium in the body, should blood calcium drop too low.
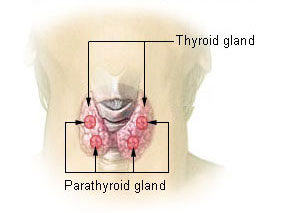
Two endocrine glands are key players in the regulation of blood calcium concentrations: the thyroid gland and parathyroid glands. The thyroid gland is a small, butterfly-shaped gland located at the base of the neck. It secretes a hormone called calcitonin. There are four parathyroid glands, each about the size of a pea and located at the back of the thyroid gland. They secrete a hormone called parathyroid hormone (PTH).
Let’s take a closer look at how the body regulates blood calcium levels. If the blood calcium concentration drops too low, the parathyroid glands release parathyroid hormone, or PTH. PTH then acts in several ways to increase blood calcium levels:
- PTH stimulates the activity of osteoclasts to release calcium from bone.
- PTH acts on the kidney to reduce the amount of calcium lost in the urine, returning more to circulation.
- PTH stimulates enzymes in the kidney that convert vitamin D to its active form, also called calcitriol. Activated vitamin D acts on the intestine to increase the absorption of calcium. Vitamin D also works together with PTH to stimulate release of calcium from the bone and reduce calcium loss in urine.
Once blood calcium levels are normal, PTH levels drop, turning off all of these mechanisms of increasing calcium.
On the other hand, if blood calcium levels become too high, the thyroid gland releases calcitonin, which inhibits the release of calcium from the bone and increases calcium excretion from the kidneys. These mechanisms help to restore normal blood calcium concentrations, after which calcitonin levels drop again.

Figure 9.5. Blood calcium (Ca 2+ ) levels are tightly regulated by PTH, vitamin D, and calcitonin.
Through these two opposing pathways—PTH and vitamin D for raising blood calcium and calcitonin for lowering blood calcium—the body can very effectively maintain blood calcium homeostasis. This system is dependent upon stored calcium in bone, which is sacrificed when needed to ensure adequate blood calcium. In the short-term, this isn’t a problem, because bone remodeling allows you to replace calcium in the bone. However, in the long-term, inadequate dietary calcium means you continuously draw down the calcium stores in your bones, resulting in declining bone mineral density and increased risk of fracture.
Calcium requirements are highest for children and adolescents, who are growing and building bones, and for older adults, who are losing bone density. The RDA for calcium for children 9 to 13 years old and teens 14 to 18 years old is 1,300 milligrams per day. The RDA for adults is 1,000 mg per day but increases to 1,200 mg per day for women ages 51 and up and for men age 71 and older.
Dietary Sources of Calcium
In the typical American diet, calcium is obtained mostly from dairy products. A slice of cheddar or Swiss cheese contains over 200 milligrams of calcium. One cup of milk contains approximately 300 milligrams of calcium, which is about a third of the RDA for calcium for most adults. Foods fortified with calcium such as cereals, soy milk and other plant-based beverages, and orange juice also provide one third or greater of the calcium RDA. Smaller amounts of calcium are naturally present in plant-based foods such as legumes, leafy greens, and nuts, and with careful planning, adequate calcium can be obtained from non-dairy sources.

Figure 9.6. Dietary sources of calcium. Examples of good sources pictured include cheese, milk, fortified soymilk, yogurt (with almond granola), edamame, and chia seeds. Source: NIH Office of Dietary Supplements and Dietary Guidelines for Americans, 2015-2020 .
Calcium bioavailability, or the amount of dietary calcium that is absorbed from the intestine into the bloodstream, can vary significantly. In general, calcium absorption is highest in infants and young children—who need relatively high amounts of calcium for building bone—and declines with age. With higher calcium intake, especially from supplements, bioavailability decreases in order to prevent excessive calcium absorption. Some chemical components of plant foods, including phytic acid (found in whole grains, beans, seeds, soy, and nuts) and oxalic acid (found in spinach, collard greens, sweet potatoes, rhubarb, and beans), bind to calcium and reduce bioavailability. Despite reduced absorption, these foods can still provide a significant amount of calcium.1
Calcium Deficiency and Toxicity
In the short-term, there are no obvious signs of calcium deficiency. This is because the body stores so much calcium in bones, and just 1% of total body calcium is required for daily functioning. If low blood calcium does occur, symptoms include muscle cramping, numbness and tingling in fingers, convulsions, lethargy, poor appetite, and abnormal heart rhythms. Without treatment, low blood calcium can lead to death.1
Much more common is a long-term calcium deficiency, resulting from a continuous draw of calcium stores from the bone. This causes osteopenia, or low bone mass, which can lead to osteoporosis if untreated. Osteoporosis significantly increases a person’s risk of fractures. Nutrition surveys in the United States show that groups at greatest risk of dietary calcium inadequacy include adolescents and older adults, especially female teens and older women.1
Too much calcium can also cause problems, although this is rarely caused by excessive intake of calcium from foods. Abnormally high activity of the parathyroid gland or a parathyroid tumor can cause high blood calcium, leading to calcification or hardening of blood vessels and soft tissue and the formation of kidney stones. High calcium intake from supplements has also been associated with increased risk of kidney stones, and in some studies, increased risk of cardiovascular disease. It can also cause constipation.1
Attributions:
- Zimmerman, M., & Snow, B. Nutrients Important for Bone Health. In An Introduction to Nutrition (v. 1.0). https://2012books.lardbucket.org/books/an-introduction-to-nutrition/index.html, CC BY-NC-SA 3.0
References:
- 1National Institutes of Health Office of Dietary Supplements. (n.d.). Calcium. Retrieved April 23, 2020, from https://ods.od.nih.gov/factsheets/Calcium-HealthProfessional/
Image Credits:
- Figure 9.4. “Thyroid and parathyroid glands” by National Cancer Institute is in the Public Domain
- Figure 9.5. “Calcium regulation” by Alice Callahan is licensed under CC BY 4.0, with kidney, bone, and intestine images by Mikael Haggstrom, in the Public Domain.
- Figure 9.6. “Dietary sources of calcium” by Alice Callahan is licensed under CC BY 4.0, with images: “Cheese” by Finite Focus is licensed under CC BY-NC 2.0; milk photo by Eiliv-Sonas Aceron on Unsplash (license information); “yogurt and granola“ by Marco Verch is licensed under CC BY 2.0; “edamame“ by Carrie T is licensed under CC BY-NC 2.0; “soymilk“ by Ian Fuller is licensed under CC BY-NC 2.0; and “chia seeds“ by Stacy Spensley is licensed under CC BY 2.0.


