11.5: Overview of Fluid and Electrolyte Balance
- Page ID
- 36365
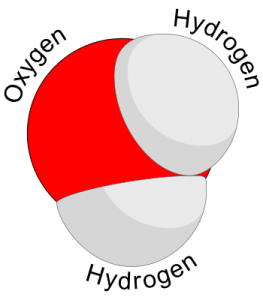
Water is made up of 2 hydrogen atoms and 1 oxygen atom. A human body is made up of mostly water. An adult consists of about 37 to 42 liters of water, or about eighty pounds. Fortunately, humans have compartmentalized tissues; otherwise we might just look like a water balloon!
Water Distribution and Composition
In the human body, water is distributed into two compartments: inside cells, called intracellular fluid (ICF), and outside cells, called extracellular fluid (ECF). Extracellular fluid includes both the fluid component of the blood (called plasma) and the interstitial fluid(IF) that surrounds all cells not in the blood.
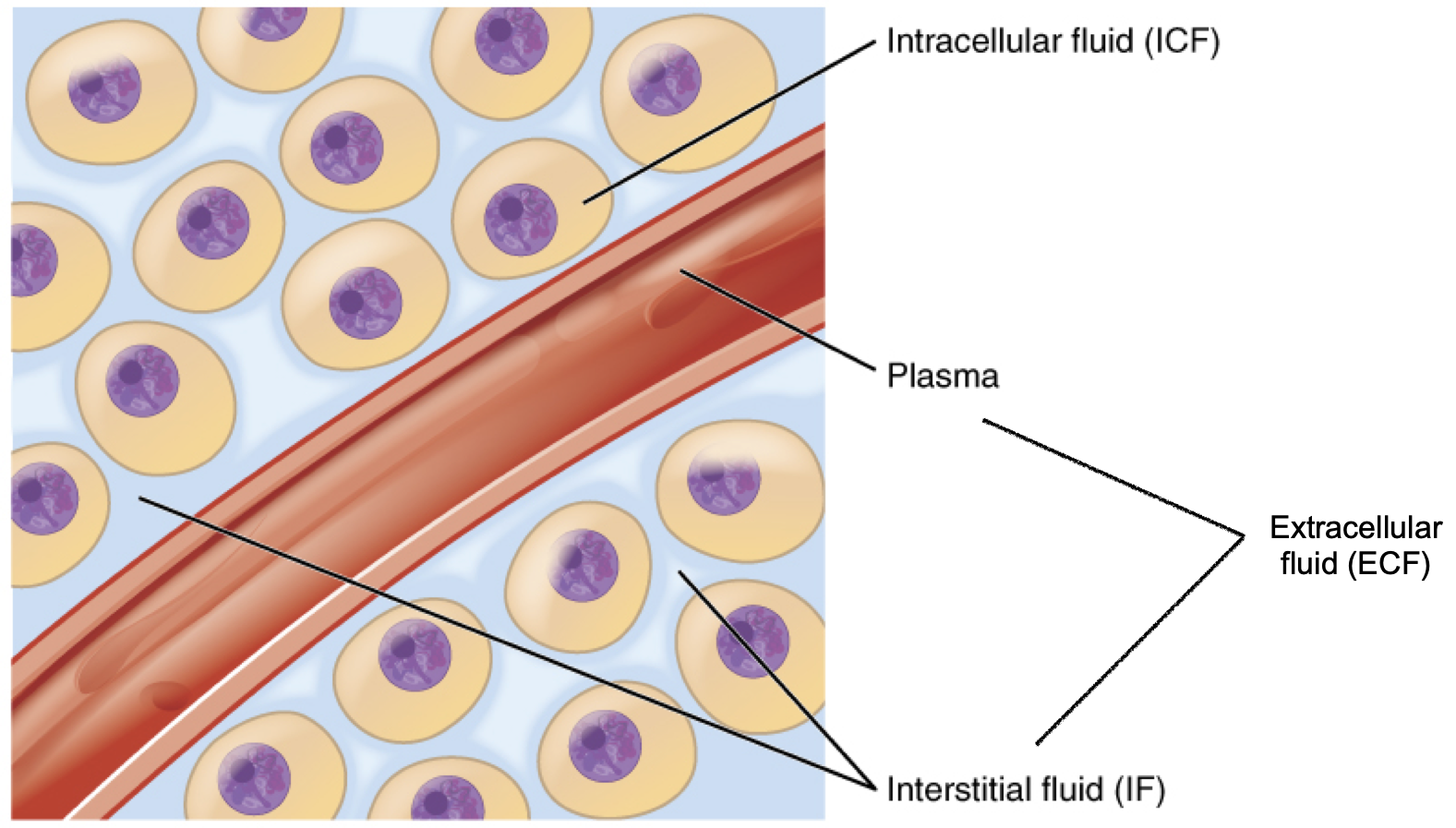
Although water makes up the largest percentage of body volume, it is not actually pure water, but rather a mixture of dissolved substances (solutes) that are critical to life. These solutes include electrolytes, substances that dissociate into charged ions when dissolved in water. For example, sodium chloride (the chemical name for table salt) dissociates into sodium (Na+) and chloride (Cl−) in water. In extracellular fluid, sodium is the major positively-charged electrolyte (or cation), and chloride (Cl−) is the major negatively-charged electrolyte (or anion). Potassium (K+) is the major cation inside cells. Together, these electrolytes are involved in many body functions, including water balance, acid-base balance, and assisting in the transmission of electrical impulses along cell membranes in nerves and muscles.
Fluid and Electrolyte Balance
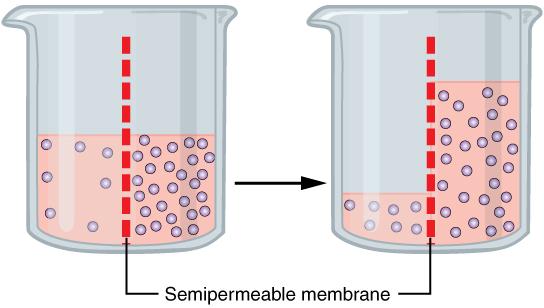
One of the essential homeostatic functions of the body is to maintain fluid and electrolyte balance within cells and their surrounding environment. Cell membranes are selectively permeable: Water can move freely through the cell membrane, while other substances, such as electrolytes, require special transport proteins, channels, and often energy. The movement of water between the intracellular and extracellular fluid happens by osmosis, which is simply the movement of water through a selectively permeable membrane from an area where solutes are less concentrated to an area where solutes are more concentrated.
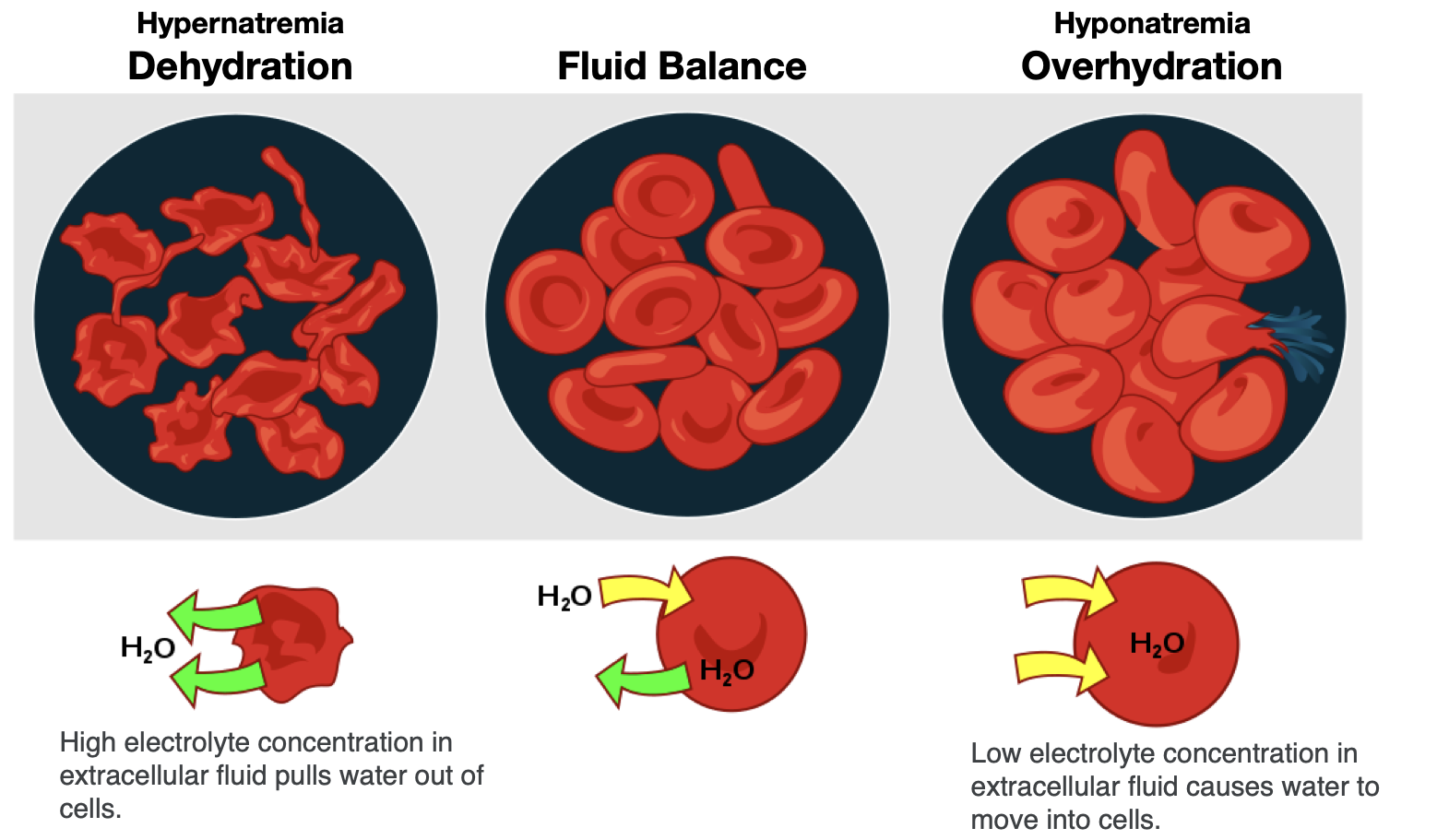
To maintain water and electrolyte balance, cells control the movement of electrolytes across their membranes, and water follows the electrolytes by osmosis. The health of the cell depends on proper fluid and electrolyte balance. If the body’s fluid and electrolyte levels change too rapidly, cells can struggle to correct the imbalance quickly enough. For example, consider a person exercising strenuously, losing water and electrolytes in the form of sweat, and drinking excessive amounts of water. The excess water dilutes the sodium in the blood, leading to hyponatremia, or low blood sodium concentrations. Sodium levels within the cells are now more concentrated, leading water to enter the cells by osmosis. As a result, the cells swell with water and can burst if the imbalance is severe and prolonged.
In contrast, the opposite situation can occur in a person exercising strenuously for a long duration with inadequate fluid intake. This can lead to dehydration and hypernatremia, or elevated blood sodium levels. The high concentration of sodium in the extracellular fluid causes water to leave cells by osmosis, making them shrink. This scenario can also occur anytime a person is dehydrated because of significant fluid loss, such as from diarrhea and/or vomiting caused by illness.
When a person becomes dehydrated, and solutes like sodium become too concentrated in the blood, the thirst response is triggered. Sensory receptors in the thirst center in the hypothalamus monitor the concentration of solutes of the blood. If blood solutes (like sodium) increase above ideal levels, the hypothalamus transmits signals that result in a conscious awareness of thirst. The hypothalamus also communicates to the kidneys to decrease water output through the urine.
The cell is able to control the movement of the two major cations, sodium and potassium, with a sodium-potassium pump (Na+/K+ pump). This pump transports sodium out of cells while moving potassium into cells.
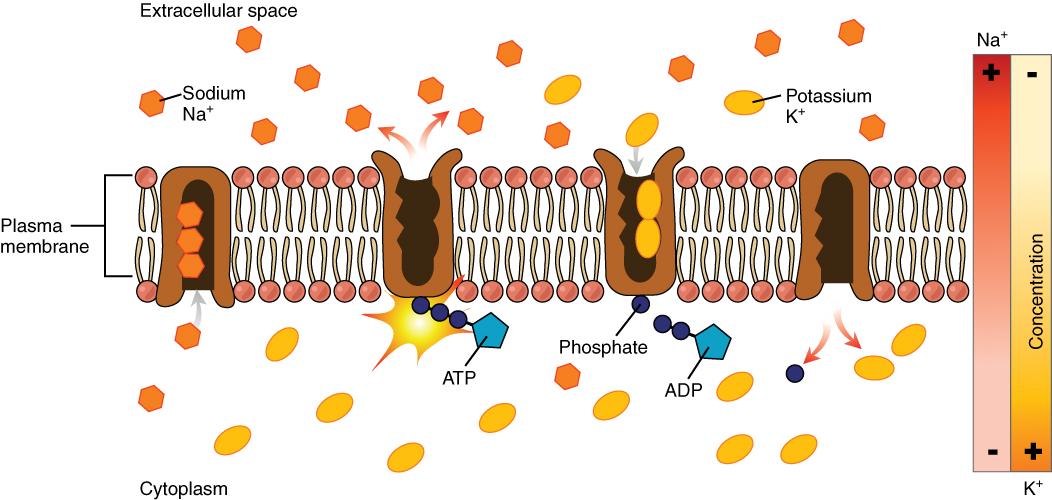
Video \(\PageIndex{1}\): “Sodium Potassium Pump,” by McGraw Hill Animations, YouTube (June 4, 2017), 2:02 minutes.
The Na+/K+ pump is an important ion pump found in the membranes of many types of cells and is particularly abundant in nerve cells. When a nerve cell is stimulated (e.g., the touch of a hand), there is an influx of sodium ions into the nerve cell. Similar to how a current moves along a wire, a sodium current moves along a nerve cell.
Stimulating a muscle contraction also involves the movement of sodium ions. For a muscle to contract, a nerve impulse travels to a muscle. The movement of the sodium current in the nerve signals the muscle cell membrane to open and sodium rushes in, creating another current that travels along the muscle and eventually leading to muscle contraction. In both nerve and muscle cells, the sodium that went in during a stimulus now has to be moved out by the sodium-potassium pump in order for the nerve and muscle cell to be stimulated again.
References
Attributions
- Alice Callahan, Heather Leonard, & Tamberly Powell, Instructors (Nutrition) at Lane Community College, Sourced from OpenOregon

