17.2.2: Long-Term Energy- Oxidative System
- Page ID
- 39169
The Oxidative System
At rest and when adequate oxygen is available to cells during glycolysis, the oxidative system makes up the bulk of energy production in the human body. The same holds true for intense exercise lasting more than 2 h after glycogen stores have been depleted; in which case the body switches to fats (lipids) as the primary source of energy.1
Please recall that dietary fats contain 9 kcal/g and therefore contribute immensely to energy metabolism in the human body; due to the almost endless reserve capacity in adipose tissue, the supply of fatty acids for energy production is virtually unlimited. In order to be used for energy in the oxidative system however, free fatty acid must be converted into acetyl Co-A (during a process called β-oxidation; please refer to segment 9.4.2 for detail).
Regardless of the energy source used, oxidation starts in the mitochondria after acetyl coenzyme-A (acetyl CoA) has been formed, and initiates a complex series of chemical reactions that allows complete oxidation of Acetyl-CoA in the Krebs cycle (also known as a citric acid cycle or tricyclic acid cycle [TCA cycle]; see Fig. 9.2.3.1 b).
Figure 9.2.3.1 The production of ATP starts during glycolysis or glycogenolysis with the formation of pyruvate (a), which is further broken down into acetyl coenzyme A (which may also be used to form a fatty acid or pyruvate) to enter the Krebs cycle (b). The hydrogen ions released in this reaction are carried to the electron transport chain where a large amount of ATP is formed (c).[Adopted and modified from 1]
The Krebs Cycle Releases High-Energy Electrons
Krebs cycle, also known as tricarboxylic acid (TCA) cycle or citric acid cycle, is regulated by negative feedback. Similar to the glycolytic pathway, it is upregulated by high concentrations of ADP and Pi whereas it is downregulated by high concentrations of ATP.2
The Krebs cycle is the third stage of energy metabolism. It results in the full oxidation of acetyl CoA (Figure 9.2.3.2) and occurs in the mitochondria. Up to this point, the individual macronutrients have followed different metabolic routes and entered the energy pathway at different points;3 now, they have all arrived at acetyl CoA.
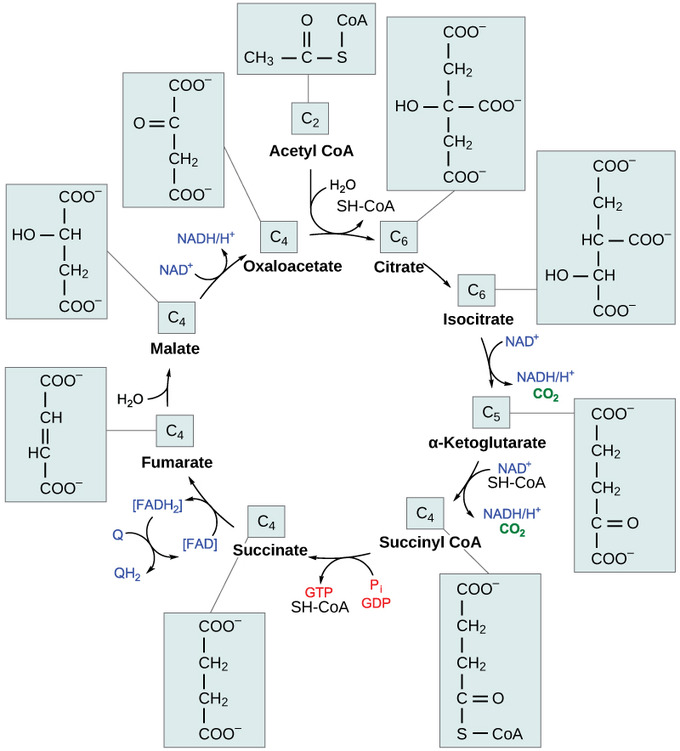
Figure 9.2.3.2 The Krebs or citric acid cycle: after conversion of pyruvate to acetyl CoA, the acetyl group from acetyl CoA is attached to a four-carbon oxaloacetate molecule to form a six-carbon citrate molecule. Through a series of steps, citrate is oxidized, releasing two carbon dioxide molecules for each acetyl group fed into the cycle. In the process, three NAD+ molecules are reduced to NADH, one FAD molecule is reduced to FADH2, and one ATP or GTP (depending on the cell type) is produced (by substrate-level phosphorylation). Because the final product of the citric acid cycle is also the first reactant, the cycle runs continuously in the presence of sufficient reactants.4
Recall that energy source molecules are carbon-containing compounds that can be oxidized or lose electrons; during each turn of the Krebs cycle, hydrogen ions, which carry high-energy electrons, are released and gathered up by NAD + , which then forms NADH + H+, and by FAD, which then forms FADH2. These electron carriers release their 'charge' in the electron transport chain (which is discussed in segment 9.3.7). In total, for each turn of the Krebs cycle, four coenzymes are reduced, along with the formation of carbon dioxide (CO2).3
Key Points in the Krebs Cycle
- The four-carbon molecule, oxaloacetate, that began the cycle is regenerated after the eight steps of the Krebs cycle.
- The eight steps of the Krebs cycle are a series of redox, dehydration, hydration, and decarboxylation reactions.
- Each turn of the cycle forms one GTP or ATP as well as three NADH molecules and one FADH2 molecule, which will be used in further steps of cellular respiration to produce ATP for the cell.
In summary, the Krebs cycle process will generate CO2 and 2 molecules of ATP.2 For more detailed biochemical information on the Krebs or Citric Acid Cycle, please see segment 9.3.6.
As the generated NADH and FADH2 molecules enter the Electron Transport Chain (see Fig. 9.2.3.1 c, and refer to segment 9.3.7 for detail), an additional 28 APT molecules are generated for a total harvest of 32-34 ATP molecule per molecule of glucose.2
Check your knowledge:
True or False: Glucose, fats and proteins can be converted into Acetyl-CoA to enter the Krebs cycle and later the electron transport chain pathways to generate energy.
Contributors
References & Links
- Sousa, A., Ribeiro, J. & Figueiredo, P. (2019). Physiological Demands in Sports Practice. 10.1007/978-3-030-10433-7_4.
- Gropper SS, Smith JL, Groff JL. (2016) Advanced nutrition and human metabolism (7th ed.). Cengage.
- Blake, J. S., Munoz, K. D., & Volpe, S. (2019). Nutrition: From Science to You (4th ed.). Pearson.
- https://bio.libretexts.org/Bookshelves/Introductory_and_General_Biology/Book%3A_General_Biology_(Boundless)/7%3A_Cellular_Respiration/7.3%3A_Oxidation_of_Pyruvate_and_the_Citric_Acid_Cycle/7.3C%3A_Citric_Acid_Cycle


