1.1: The Basics
- Page ID
- 20961
Nutrition can be defined as the science of the action of food, beverages, and their components in biological systems. A nutrient is a compound that provides a needed function in the body. Nutrients can be further classified based on the amount needed in the body.
- Macronutrients: nutrients needed in larger amounts
- Micronutrients: nutrients needed in smaller amounts (but still important)
The following table lists the different macronutrients and micronutrients.
| Macronutrients | Micronutrients |
|---|---|
| Carbohydrates | Vitamins |
| Proteins | Minerals |
| Lipids | |
| Water |
- Carbohydrates: The name carbohydrate means "hydrated carbon", or carbon with water. Thus, it isn't a surprise that carbohydrates are made up of carbon, hydrogen, and oxygen. Sucrose (table sugar) is an example of a commonly consumed carbohydrate. Some dietary examples of carbohydrates are whole-wheat bread, oatmeal, rice, sugary snacks/drinks, and pasta.
- Proteins: Proteins are also made up of carbon, hydrogen, and oxygen, but they also contain nitrogen. Several dietary sources of proteins include nuts, beans/legumes, skim milk, egg whites, and meat.
- Lipids: Lipids consist of fatty acids, triglycerides, phospholipids, and sterols (i.e. cholesterol). Lipids are also composed of carbon, hydrogen, and oxygen. Some dietary sources of lipids include oils, butter, and egg yolks.
- Water: Water is made up of hydrogen and oxygen (\(\ce{H2O}\)) and is the only macronutrient that doesn't provide energy.
- Vitamins: Organic compounds that are essential for normal physiologic processes in the body.
- Minerals: Elements (think periodic table) that are essential for normal physiological processes in the body.
Calories (Food Energy)
Food energy is measured in kilocalories (kcals), commonly referred to as calories by the general public. The general public “calorie” term is incorrect (most do not know or understand the difference between the kilocalorie and calorie terms), but it is important to understand what the term calorie represents when it is used in this way. A kilocalorie is the amount of energy needed to raise 1 kilogram of water 1 degree Celsius. A food’s kilocalories are determined by putting the food into a bomb calorimeter and determining the energy output (energy = heat produced). The link below is to an animation of a bomb calorimeter.
Among the nutrients, the amount of kilocalories per gram that each provide are shown below.
| Energy (kcal/g) | No Energy |
|---|---|
| Carbohydrates (4) | Vitamins |
| Proteins (4) | Minerals |
| Lipids (9) | Water |
As can be seen, only carbohydrates, proteins, and lipids provide energy. However, there is another energy source in the diet that is not a nutrient…...alcohol. Just to re-emphasize, alcohol is NOT a nutrient! But it does provide energy.
Below is a list of energy sources in the diet from lowest calories per gram to the highest calories per gram. Knowing these numbers allows a person to calculate/estimate the amount of calories the food contains if you know the grams of the different energy sources.
- Energy Sources (kcal/g)
- Carbohydrates 4
- Protein 4
- Alcohol 7
- Lipids 9
Phytochemicals, Zoochemicals & Functional Foods
Beyond macronutrients and micronutrients, there is a lot of interest in non-nutritive compounds found in foods that may be either beneficial or detrimental to health.
Phytochemicals
Phytochemicals are compounds in plants (phyto) that are believed to provide health benefits beyond the traditional nutrients. One example is lycopene in tomatoes, which is thought to potentially decrease the risk of some cancers (in particular prostate cancer). Diets rich in fruits and vegetables have been associated with decreased risk of chronic diseases. Many fruits and vegetables are rich in phytochemicals, leading some to hypothesize that phytochemicals are responsible for the decreased risk of chronic diseases. The role that phytochemicals play in health is still in the early stages of research, relative to other areas of nutrition such as micronutrients. The following link contain good information on phytonutrients if you are interested in learning more about them.
Zoochemicals
Zoochemicals are the animal equivalent of phytochemicals in plants. They are compounds in animals that are believed to provide health benefits beyond the traditional nutrients that food contains. Hopefully the name is pretty easy to remember because you can find animals at a zoo. Some compounds can be both phytochemicals and zoochemicals. An example of compounds that can be classified as both are the yellow carotenoids lutein and zeaxanthin. Kale, spinach, and corn contain phytochemicals and are good sources of lutein and zeaxanthin. Whereas egg yolks contain zoochemicals and are also a good source of these carotenoids.
Functional Foods
There are a number of definitions of functional foods. Functional foods are generally understood to be a food, or a food ingredient, which may provide a health benefit beyond the traditional nutrients (macronutrients and micronutrients) it contains. Functional foods are often a rich source of a phytochemical or zoochemical, or contain more of a certain nutrient than a normal food.
The Scientific Method
The basis of what we know about nutrition is derived from research and the scientific method underlies how research is conducted. The following figure shows the steps in the scientific method.
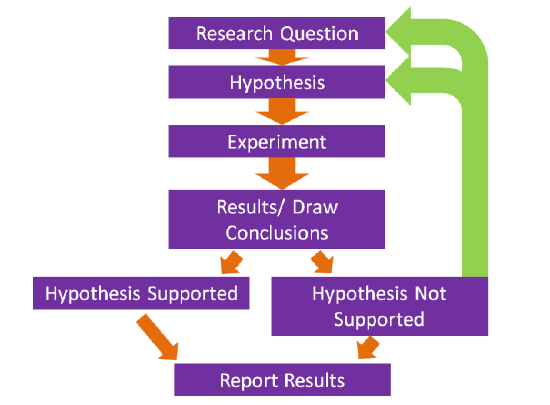
Steps in the Scientific Method
- The first step is to come up with a scientific or research question that you are interested in investigating.
- Based on your research question, a hypothesis or an educated guess is formulated.
- The next step is to design and conduct the experiment. A good design should take into account what has been done previously. Thus, a thorough review of methods and results published previously should be undertaken. This will help prevent making the same mistakes and save a lot of time conducting the research.
- Perform the experiment/research and collect results and draw conclusions.
- If the hypothesis is not supported, then a new hypothesis/research question should be created and a new experiment be conducted.
- Ultimately, researchers hope to publish their research in peer-reviewed journals.


