17.11: Amino Acids, Proteins, and Enzymes (Exercises)
- Last updated
- Save as PDF
- Page ID
- 15379
Concept Review Exercises
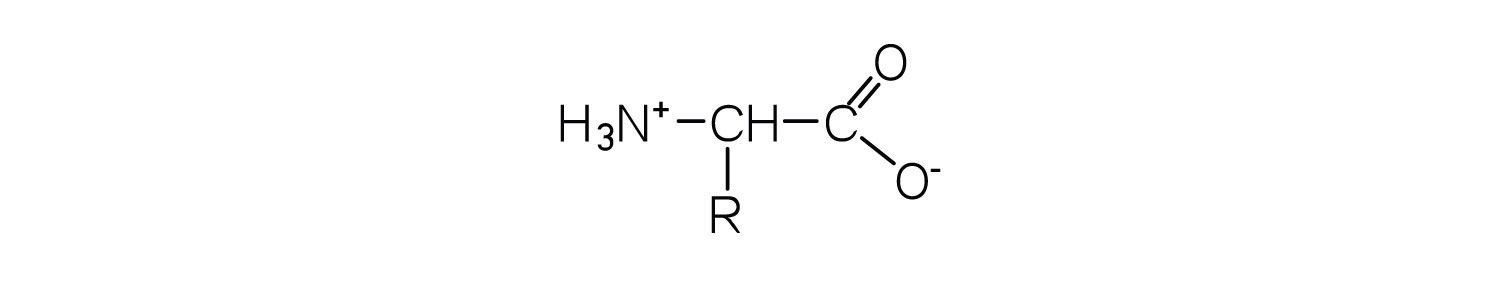
- What is the general structure of an α-amino acid?
- Identify the amino acid that fits each description.
- also known as aspartate
- almost as strong a base as sodium hydroxide
- does not have a chiral carbon
-
-
- aspartic acid
- arginine
- glycine
- Write the side chain of each amino acid.
- serine
- arginine
- phenylalanine
- Write the side chain of each amino acid.
- aspartic acid
- methionine
- valine
- Draw the structure for each amino acid.
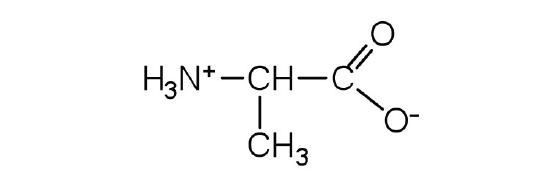
- alanine
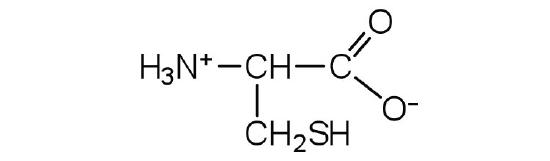
- cysteine
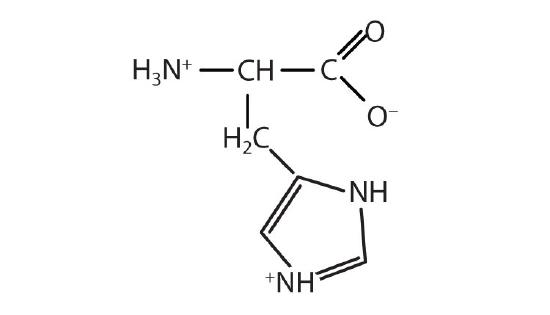
- histidine
- Draw the structure for each amino acid.
- threonine
- glutamic acid
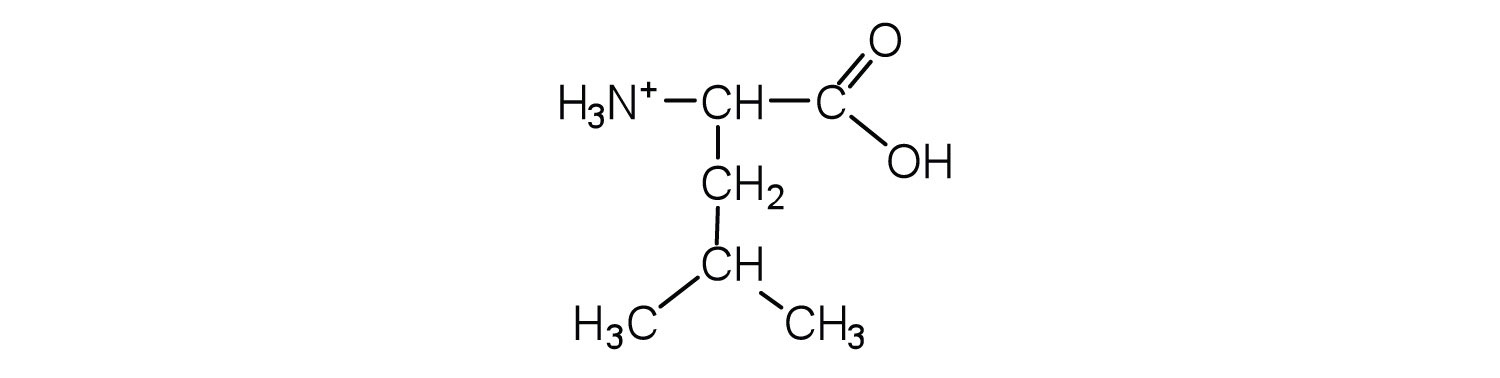
- leucine
- Identify an amino acid whose side chain contains a(n)
- amide functional group.
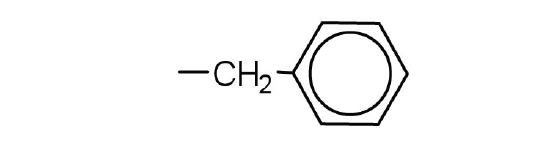
- aromatic ring.
- carboxyl group.
- Identify an amino acid whose side chain contains a(n)
- OH group
- branched chain
- amino group
-
- CH2OH−
-
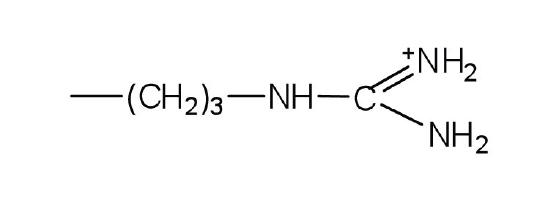
-
-
-
-
- asparagine or glutamine
- phenylalanine, tyrosine, or tryptophan
- aspartic acid or glutamic acid
Concept Review Exercises
- Define each term.
- zwitterion
- isoelectric point
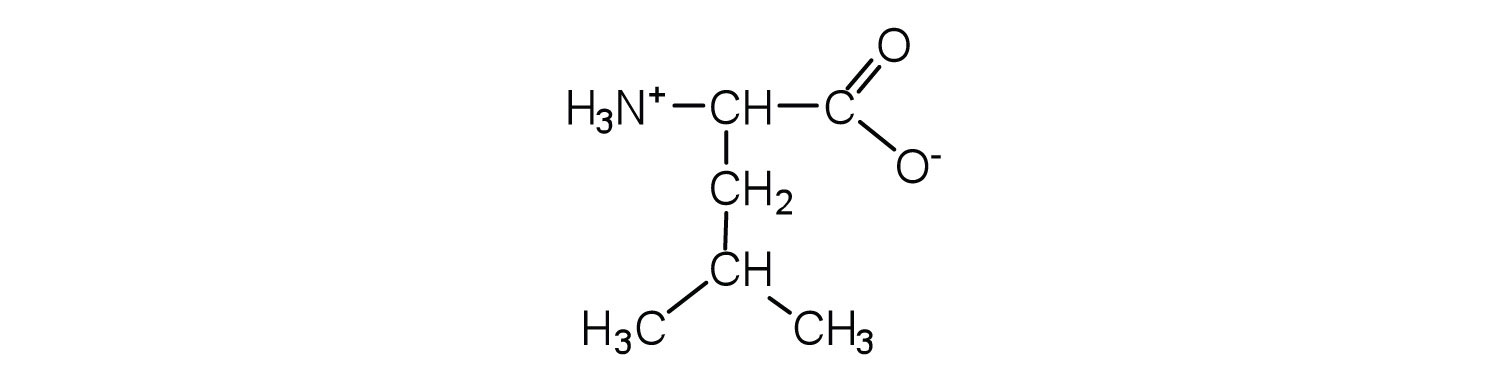
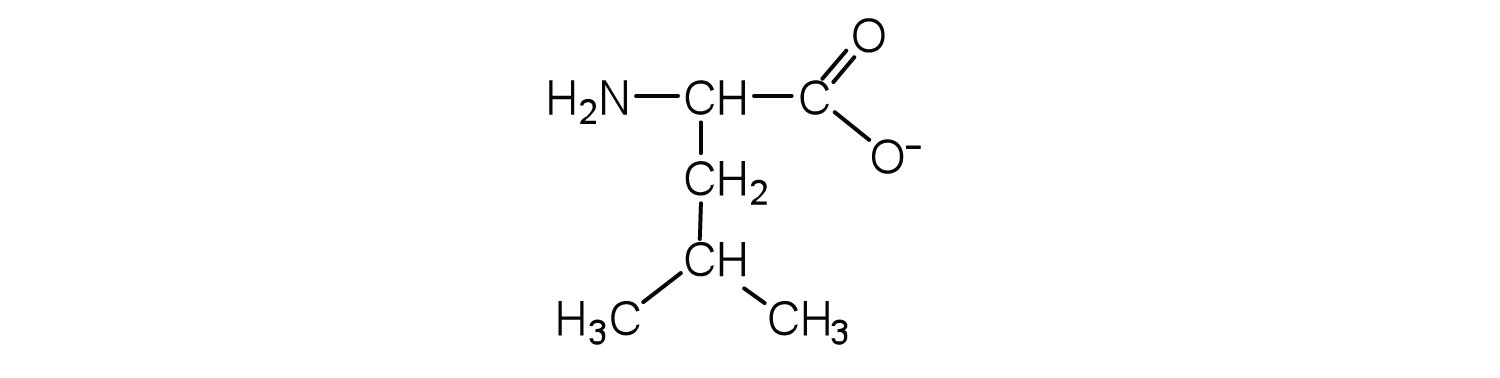
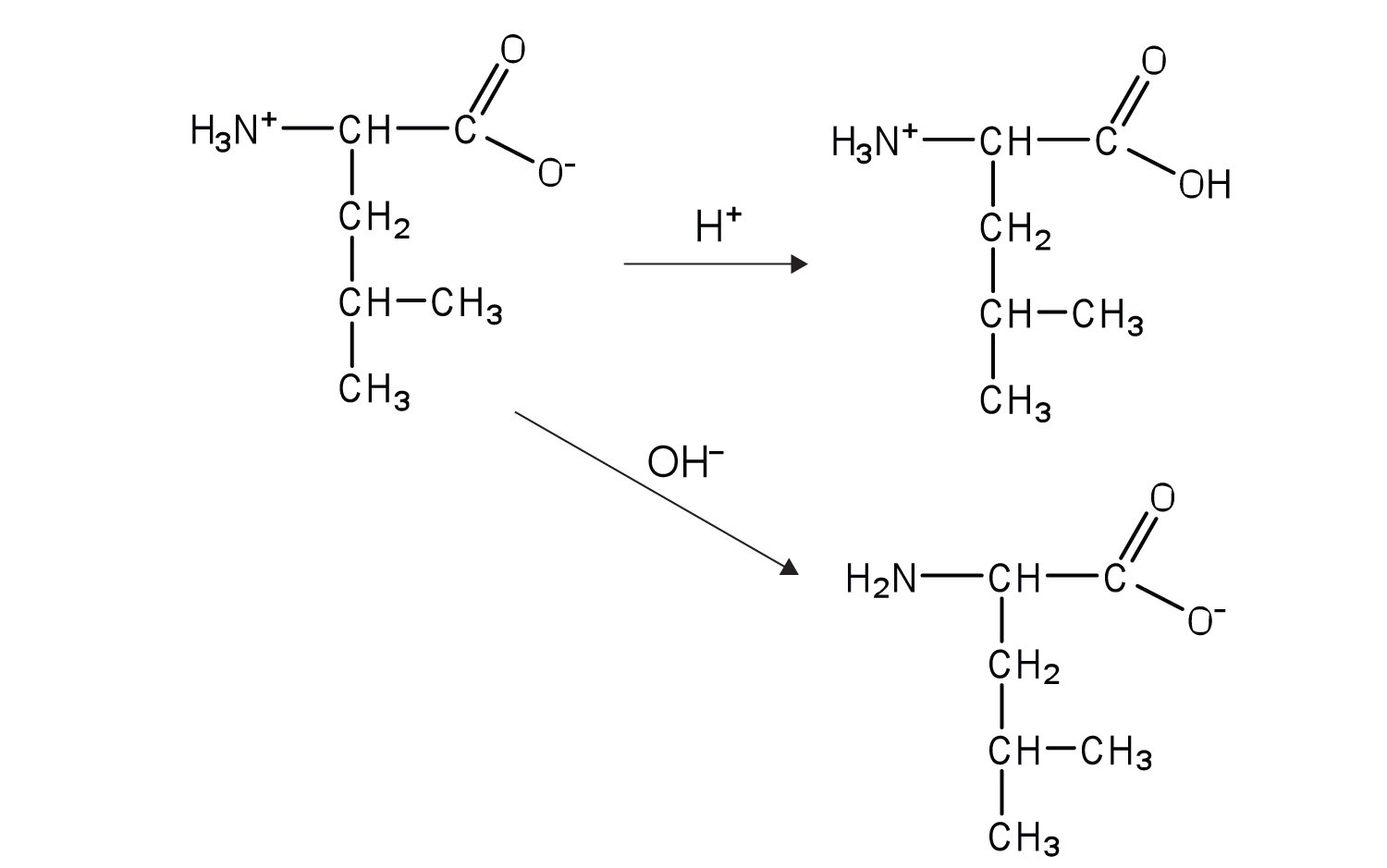
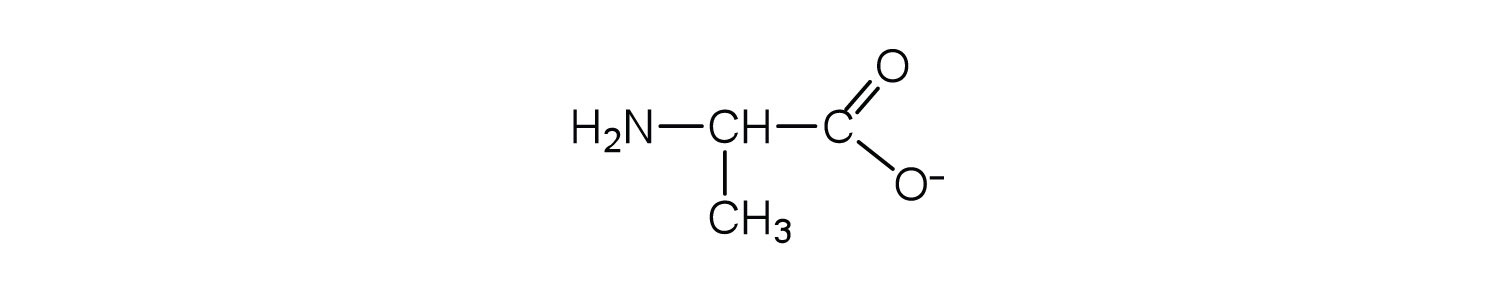
- Draw the structure for the anion formed when alanine (at neutral pH) reacts with a base.
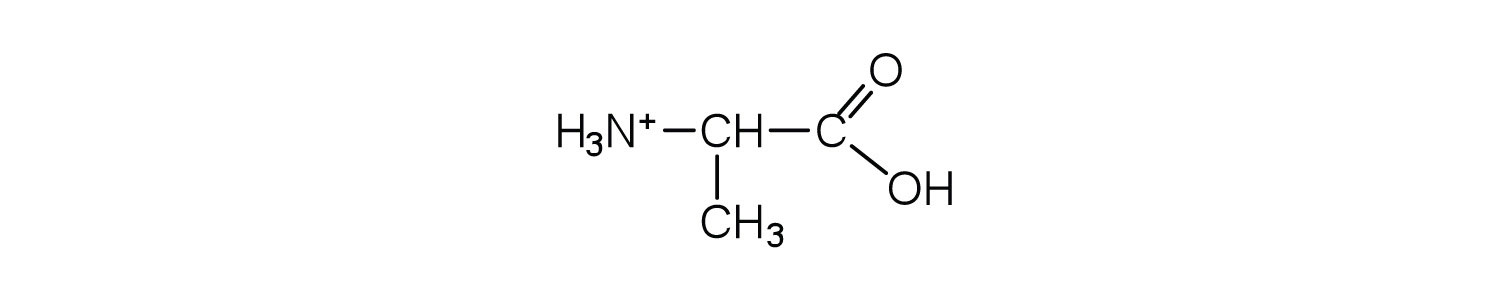
- Draw the structure for the cation formed when alanine (at neutral pH) reacts with an acid.
Answers
-
- an electrically neutral compound that contains both negatively and positively charged groups
- the pH at which a given amino acid exists in solution as a zwitterion
-
-
Exercises
- Draw the structure of leucine and determine the charge on the molecule in a(n)
- acidic solution (pH = 1).
- neutral solution (pH = 7).
- a basic solution (pH = 11)
- Draw the structure of isoleucine and determine the charge on the molecule in a(n)
- acidic solution (pH = 1).
- neutral solution (pH = 7).
- basic solution (pH = 11).
Answer
Concept Review Exercises
- Distinguish between the N-terminal amino acid and the C-terminal amino acid of a peptide or protein.
- Describe the difference between an amino acid and a peptide.
- Amino acid units in a protein are connected by peptide bonds. What is another name for the functional group linking the amino acids?
Answers
- The N-terminal end is the end of a peptide or protein whose amino group is free (not involved in the formation of a peptide bond), while the C-terminal end has a free carboxyl group.
- A peptide is composed of two or more amino acids. Amino acids are the building blocks of peptides.
- amide bond
Exercises
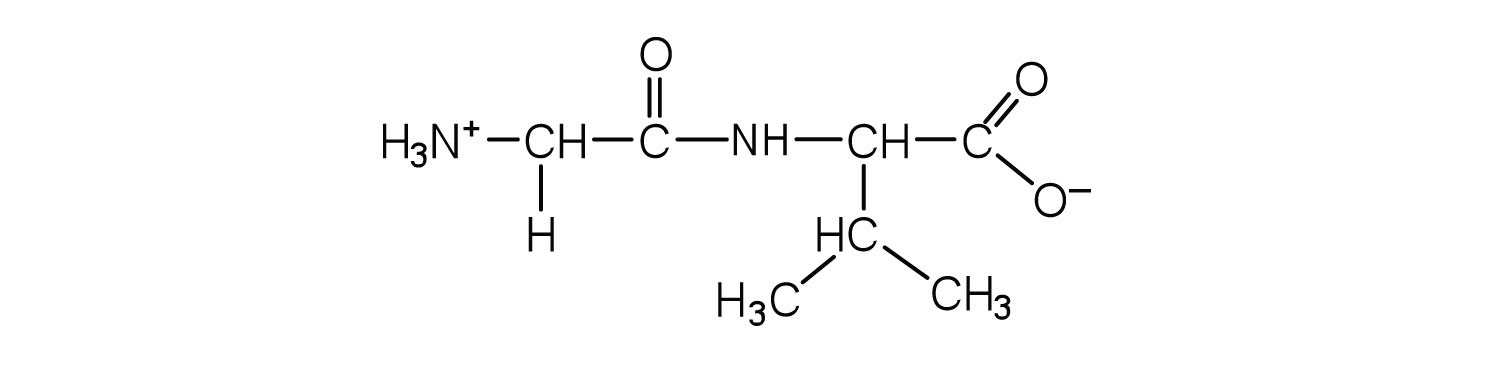
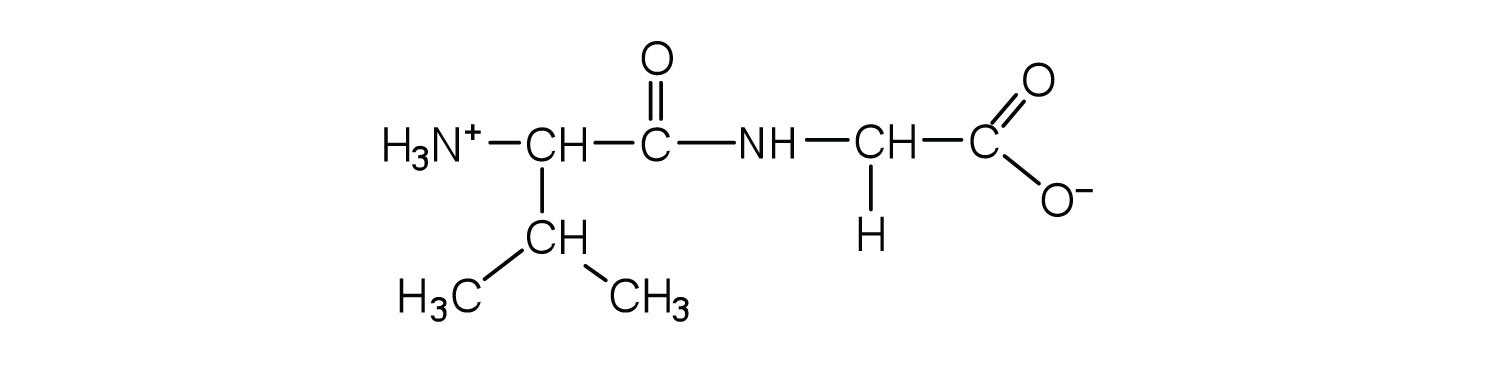
- Draw the structure for each peptide.
- gly-val
- val-gly
- Draw the structure for cys-val-ala.
- Identify the C- and N-terminal amino acids for the peptide lys-val-phe-gly-arg-cys.
- Identify the C- and N-terminal amino acids for the peptide asp-arg-val-tyr-ile-his-pro-phe.
Answers
- C-terminal amino acid: cys; N-terminal amino acid: lys
Concept Review Exercises
- What is the predominant attractive force that stabilizes the formation of secondary structure in proteins?
- Distinguish between the tertiary and quaternary levels of protein structure.
- Briefly describe four ways in which a protein could be denatured.
Answers
- hydrogen bonding
- Tertiary structure refers to the unique three-dimensional shape of a single polypeptide chain, while quaternary structure describes the interaction between multiple polypeptide chains for proteins that have more than one polypeptide chain.
- (1) heat a protein above 50°C or expose it to UV radiation; (2) add organic solvents, such as ethyl alcohol, to a protein solution; (3) add salts of heavy metal ions, such as mercury, silver, or lead; and (4) add alkaloid reagents such as tannic acid
Exercises
- Classify each protein as fibrous or globular.
- albumin
- myosin
- fibroin
- Classify each protein as fibrous or globular.
- hemoglobin
- keratin
- myoglobin
- What name is given to the predominant secondary structure found in silk?
- What name is given to the predominant secondary structure found in wool protein?
- A protein has a tertiary structure formed by interactions between the side chains of the following pairs of amino acids. For each pair, identify the strongest type of interaction between these amino acids.
- aspartic acid and lysine
- phenylalanine and alanine
- serine and lysine
- two cysteines
- A protein has a tertiary structure formed by interactions between the side chains of the following pairs of amino acids. For each pair, identify the strongest type of interaction between these amino acids.
- valine and isoleucine
- asparagine and serine
- glutamic acid and arginine
- tryptophan and methionine
- What level(s) of protein structure is(are) ordinarily disrupted in denaturation? What level(s) is(are) not?
- Which class of proteins is more easily denatured—fibrous or globular?
Answers
-
- globular
- fibrous
- fibrous
- β-pleated sheet
-
- ionic bonding
- dispersion forces
- dispersion forces
- disulfide linkage
- Protein denaturation disrupts the secondary, tertiary, and quaternary levels of structure. Only primary structure is unaffected by denaturation.
Concept Review Exercise
In the small intestine, sucrose is hydrolyzed to form glucose and fructose in a reaction catalyzed by sucrase.
- Identify the substrate in this reaction.
- Name the enzyme.
Answers
- sucrose
- sucrase
Exercises
- Identify the substrate catalyzed by each enzyme.
- lactase
- cellulase
- peptidase
- Identify the substrate catalyzed by each enzyme.
- lipase
- amylase
- maltase
- Identify each type of enzyme.
- decarboxylase
- protease
- transaminase
- Identify each type of enzyme.
- dehydrogenase
- isomerase
- lipase
Answers
-
- lactose
- cellulose
- peptides
-
- lyase
- hydrolase
- transferase
Concept Review Exercises
- Distinguish between the lock-and-key model and induced-fit model of enzyme action.
- Which enzyme has greater specificity—urease or carboxypeptidase? Explain.
Answers
- The lock-and-key model portrays an enzyme as conformationally rigid and able to bond only to substrates that exactly fit the active site. The induced fit model portrays the enzyme structure as more flexible and is complementary to the substrate only after the substrate is bound.
- Urease has the greater specificity because it can bind only to a single substrate. Carboxypeptidase, on the other hand, can catalyze the removal of nearly any amino acid from the carboxyl end of a peptide or protein.
Exercises
- What type of interaction would occur between each group present on a substrate molecule and a functional group of the active site in an enzyme?
- COOH
- NH3+
- OH
- CH(CH3)2
- What type of interaction would occur between each group present on a substrate molecule and a functional group of the active site in an enzyme?
- SH
- NH2
- C6H5
- COO−
- For each functional group in Exercise 1, suggest an amino acid whose side chain might be in the active site of an enzyme and form the type of interaction you identified.
- For each functional group in Exercise 2, suggest an amino acid whose side chain might be in the active site of an enzyme and form the type of interaction you identified.
Answers
-
- hydrogen bonding
- ionic bonding
- hydrogen bonding
- dispersion forces
-
- The amino acid has a polar side chain capable of engaging in hydrogen bonding; serine (answers will vary).
- The amino acid has a negatively charged side chain; aspartic acid (answers will vary).
- The amino acid has a polar side chain capable of engaging in hydrogen bonding; asparagine (answers will vary).
- The amino acid has a nonpolar side chain; isoleucine (answers will vary).
Concept Review Exercises
- The concentration of substrate X is low. What happens to the rate of the enzyme-catalyzed reaction if the concentration of X is doubled?
- What effect does an increase in the enzyme concentration have on the rate of an enzyme-catalyzed reaction?
Answers
- If the concentration of the substrate is low, increasing its concentration will increase the rate of the reaction.
- An increase in the amount of enzyme will increase the rate of the reaction (provided sufficient substrate is present).
Exercises
- In non-enzyme-catalyzed reactions, the reaction rate increases as the concentration of reactant is increased. In an enzyme-catalyzed reaction, the reaction rate initially increases as the substrate concentration is increased but then begins to level off, so that the increase in reaction rate becomes less and less as the substrate concentration increases. Explain this difference.
- Why do enzymes become inactive at very high temperatures?
- An enzyme has an optimum pH of 7.4. What is most likely to happen to the activity of the enzyme if the pH drops to 6.3? Explain.
- An enzyme has an optimum pH of 7.2. What is most likely to happen to the activity of the enzyme if the pH increases to 8.5? Explain.
Answers
- In an enzyme-catalyzed reaction, the substrate binds to the enzyme to form an enzyme-substrate complex. If more substrate is present than enzyme, all of the enzyme binding sites will have substrate bound, and further increases in substrate concentration cannot increase the rate.
- The activity will decrease; a pH of 6.3 is more acidic than 7.4, and one or more key groups in the active site may bind a hydrogen ion, changing the charge on that group.
Concept Review Exercises
- What is the difference between a cofactor and a coenzyme?
- How are vitamins related to coenzymes?
Answers
- A coenzyme is one type of cofactor. Coenzymes are organic molecules required by some enzymes for activity. A cofactor can be either a coenzyme or an inorganic ion.
- Coenzymes are synthesized from vitamins.
Exercises
- Identify each vitamin as water soluble or fat soluble.
- vitamin D
- vitamin C
- vitamin B12
- Identify each vitamin as water soluble or fat soluble.
- niacin
- cholecalciferol
- biotin
- What vitamin is needed to form each coenzyme?
- pyridoxal phosphate
- flavin adenine dinucleotide
- coenzyme A
- nicotinamide adenine dinucleotide
- What coenzyme is formed from each vitamin?
- niacin
- thiamine
- cyanocobalamin
- pantothenic acid
- What is the function of each vitamin or coenzyme?
- flavin adenine dinucleotide
- vitamin A
- biotin
- What is the function of each vitamin or coenzyme?
- vitamin K
- pyridoxal phosphate
- tetrahydrofolate
Answers
-
- fat soluble
- water soluble
- water soluble
-
- vitamin B6 or pyridoxine
- vitamin B2 or riboflavin
- pantothenic acid
- vitamin B3 or niacin
-
- needed by enzymes that catalyze oxidation-reduction reactions in which two hydrogen atoms are transferred
- needed for the formation of vision pigments
- needed by enzymes that catalyze carboxylation reactions
Concept Review Exercises
- What are the characteristics of an irreversible inhibitor?
- In what ways does a competitive inhibitor differ from a noncompetitive inhibitor?
Answers
- It inactivates an enzyme by bonding covalently to a particular group at the active site.
- A competitive inhibitor structurally resembles the substrate for a given enzyme and competes with the substrate for binding at the active site of the enzyme. A noncompetitive inhibitor binds at a site distinct from the active site and can bind to either the free enzyme or the enzyme-substrate complex.
Exercises
- What amino acid is present in the active site of all enzymes that are irreversibly inhibited by nerve gases such as DIFP?
- Oxaloacetate (OOCCH2COCOO) inhibits succinate dehydrogenase. Would you expect oxaloacetate to be a competitive or noncompetitive inhibitor? Explain.
Answer
- serine
Additional Exercises
-
Draw the structure of the amino acid γ-aminobutyric acid (GABA). Would you expect to find GABA in the amino acid sequence of a protein? Explain.
-
Draw the structure of the amino acid homocysteine (R group = CH2CH2SH). Would you expect to find homocysteine in the amino acid sequence of a protein? Justify your answer.
-
Write equations to show how leucine can act as a buffer (that is, how it can neutralize added acid or base).
-
Write equations to show how isoleucine can act as a buffer (that is, how it can neutralize added acid or base).
-
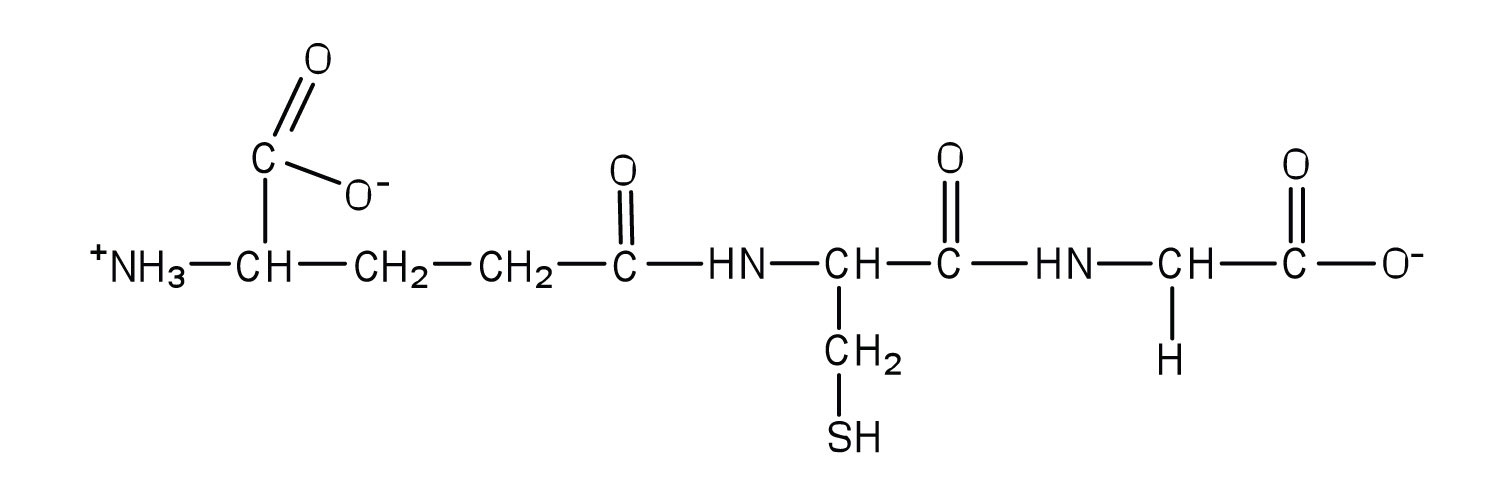
Glutathione (γ-glutamylcysteinylglycine) is a tripeptide found in all cells of higher animals. It contains glutamic acid joined in an unusual peptide linkage involving the carboxyl group of the R group (known as γ-carboxyl group), rather than the usual carboxyl group (the α-carboxyl group). Draw the structure of glutathione.
-
Draw the structure of the pentapeptide whose sequence is arg-his-gly-leu-asp. Identify which of the amino acids have R groups that can donate or gain hydrogen ions.
-
Bradykinin is a peptide hormone composed of nine amino acids that lowers blood pressure. Its primary structure is arg-pro-pro-gly-phe-ser-pro-phe-arg. Would you expect bradykinin to be positively charged, negatively charged, or neutral at a pH of 6.0? Justify your answer.
-
One of the neurotransmitters involved in pain sensation is a peptide called substance P, which is composed of 11 amino acids and is released by nerve-cell terminals in response to pain. Its primary structure is arg-pro-lys-pro-gln-gln-phe-phe-gly-leu-met. Would you expect this peptide to be positively charged, negatively charged, or neutral at a pH of 6.0? Justify your answer.
-
Carbohydrates are incorporated into glycoproteins. Would you expect the incorporation of sugar units to increase or decrease the solubility of a protein? Justify your answer.
-
Some proteins have phosphate groups attached through an ester linkage to the OH groups of serine, threonine, or tyrosine residues to form phosphoproteins. Would you expect the incorporation of a phosphate group to increase or decrease the solubility of a protein? Justify your answer.
-
Refer to Table 18.5 and determine how each enzyme would be classified.
- the enzyme that catalyzes the conversion of ethanol to acetaldehyde
- the enzyme that catalyzes the breakdown of glucose 6-phosphate to glucose and inorganic phosphate ion (water is also a reactant in this reaction)
-
Refer to Table 18.5 and determine how each enzyme would be classified.
-
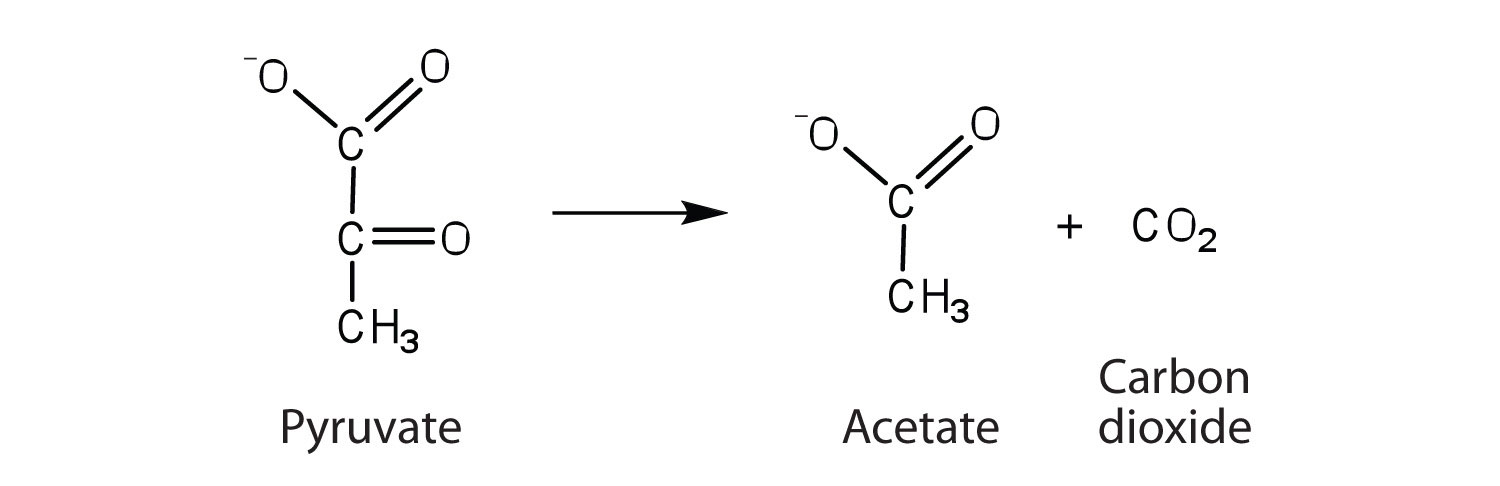
the enzyme that catalyzes the removal of a carboxyl group from pyruvate to form acetate
-
the enzyme that catalyzes the rearrangement of 3-phosphoglycerate to form 2-phosphoglycerate
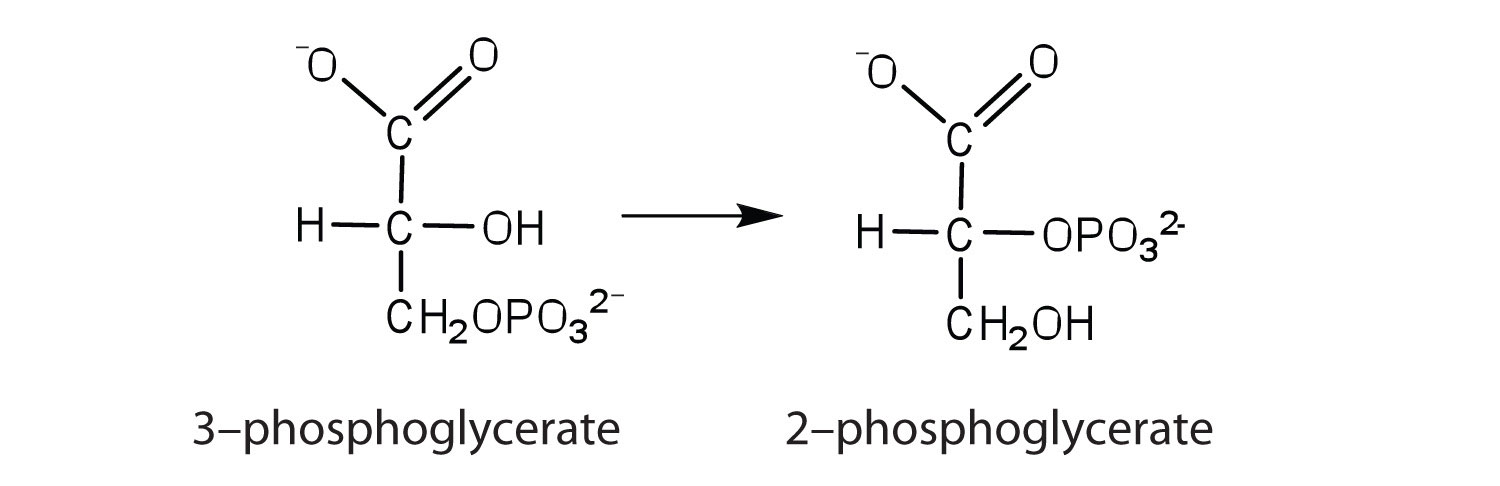
-
-
The enzyme lysozyme has an aspartic acid residue in the active site. In acidic solution, the enzyme is inactive, but activity increases as the pH rises to around 6. Explain why.
-
The enzyme lysozyme has a glutamic acid residue in the active site. At neutral pH (6–7), the enzyme is active, but activity decreases as the pH rises. Explain why.
-
The activity of a purified enzyme is measured at a substrate concentration of 1.0 μM and found to convert 49 μmol of substrate to product in 1 min. The activity is measured at 2.0 μM substrate and found to convert 98 μmol of substrate to product/minute.
- When the substrate concentration is 100 μM, how much substrate would you predict is converted to product in 1 min? What if the substrate concentration were increased to 1,000 μM (1.0 mM)?
- The activities actually measured are 676 μmol product formed/minute at a substrate concentration of 100 μM and 698 μmol product formed/minute at 1,000 μM (1.0 mM) substrate. Is there any discrepancy between these values and those you predicted in Exercise 15a? Explain.
-
A patient has a fever of 39°C. Would you expect the activity of enzymes in the body to increase or decrease relative to their activity at normal body temperature (37°C)?
-
Using your knowledge of factors that influence enzyme activity, describe what happens when milk is pasteurized.
Answers
-
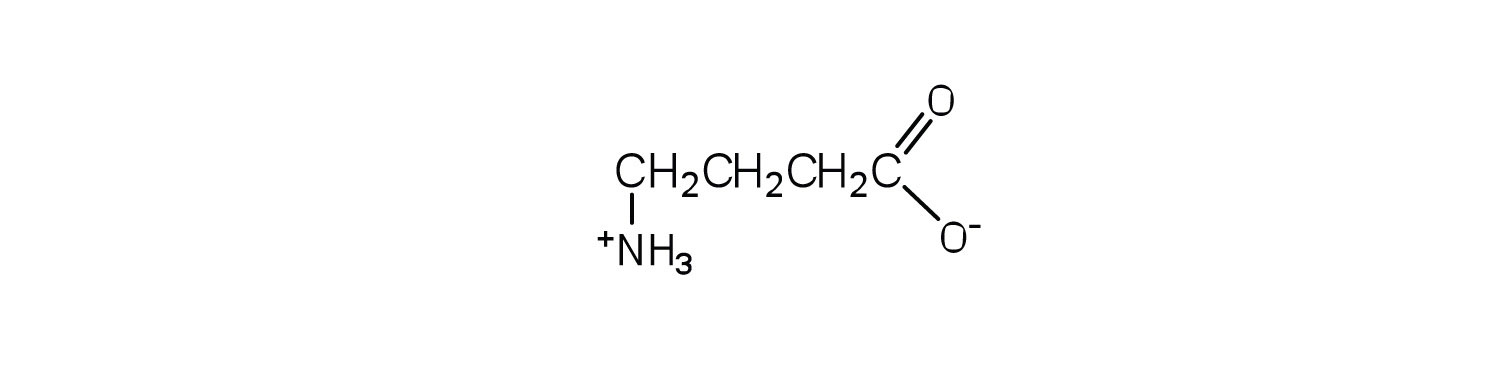
This amino acid would not be found in proteins because it is not an α-amino acid.
-
Bradykinin would be positively charged; all of the amino acids, except for arginine, have R groups that do not become either positively or negatively charged. The two arginines are R groups that are positively charged at neutral pH, so the peptide would have an overall positive charge.
-
Carbohydrates have many OH groups attached, which can engage in hydrogen bonding with water, which increases the solubility of the proteins.
-
- oxidoreductase
- hydrolase
-
The enzyme is active when the carboxyl group in the R group of aspartic acid does not have the hydrogen attached (forming COO–); the hydrogen is removed when the pH of the solution is around pH 6 or higher.
-
- at 100 μM, you would predict that the rate would increase 100 times to 4,900 μmol of substrate to product in 1 min; at 1.0 mM, you would predict an increase to 49,000 μmol of substrate to product in 1 min.
- There is a great discrepancy between the predicted rates and actual rates; this occurs because the enzyme becomes saturated with substrate, preventing a further increase in the rate of the reaction (the reaction is no longer linear with respect to substrate concentration because it is at very low concentrations).
-
When milk is pasteurized, it is heated to high temperatures. These high temperatures denature the proteins in bacteria, so they cannot carry out needed functions to grow and multiply.