15.4: Electrolytes
- Page ID
- 52499
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Electrolytes play an important role in bodily functions and fluid regulation. There is a very narrow target range for normal electrolyte values, and slight abnormalities can have devastating consequences. For this reason, it is crucial to understand normal electrolyte ranges, causes of electrolyte imbalances, signs and symptoms of imbalances, and appropriate treatments.
Sodium
Sodium levels in the blood typically range from 136-145 mEq/L.[1] Refer to each agency’s normal reference range on the lab report. Sodium is the most abundant electrolyte in the extracellular fluid (ECF) and is maintained by the sodium-potassium pump. Sodium plays an important role in maintaining adequate fluid balance in the intravascular and interstitial spaces. See the “Fluid and Electrolyte Regulation” section of this chapter for more information about how the body regulates sodium and water balance.
Hypernatremia refers to an elevated sodium level in the blood. Typically, hypernatremia is caused by excess water loss due to lack of fluid intake, vomiting, or diarrhea. As you recall, elevated sodium levels in the blood cause the osmotic movement of water out of the cells to dilute the blood. This causes the body’s cells to shrink, referred to as cellular dehydration. This fluid shift can have a significant impact on various organs within the body and is especially notable in the patient’s neurological function. As fluid shifts out of the brain cells, the nurse may notice symptoms such as confusion, irritability, lethargy, and even seizures. Other signs and symptoms of hypernatremia include severe thirst and sticky mucous membranes. See Figure 15.12[2]for an illustration of a patient with severe thirst due to hypernatremia. Treatment for hypernatremia includes decreasing sodium intake, increasing oral water intake, and rehydrating with a hypotonic IV solution.[3],[4]
Hyponatremia refers to a decreased sodium level in the blood. Hyponatremia can be caused by excess water intake or excessive administration of hypotonic IV solutions. For example, a marathon runner who only rehydrates with water (without other fluids with solutes like Gatorade) can develop hyponatremia. As with hypernatremia, altered sodium levels often cause neurological symptoms due to the movement of water into brain cells, causing them to swell. Symptoms of hyponatremia are headache, confusion, seizures, and coma. Treatment for hyponatremia depends on the cause and often consists of limiting water intake or discontinuing administration of hypotonic IV fluids. If hyponatremia is severe, a hypertonic IV saline solution may be prescribed to gradually raise the patient’s sodium level.[5]
Potassium
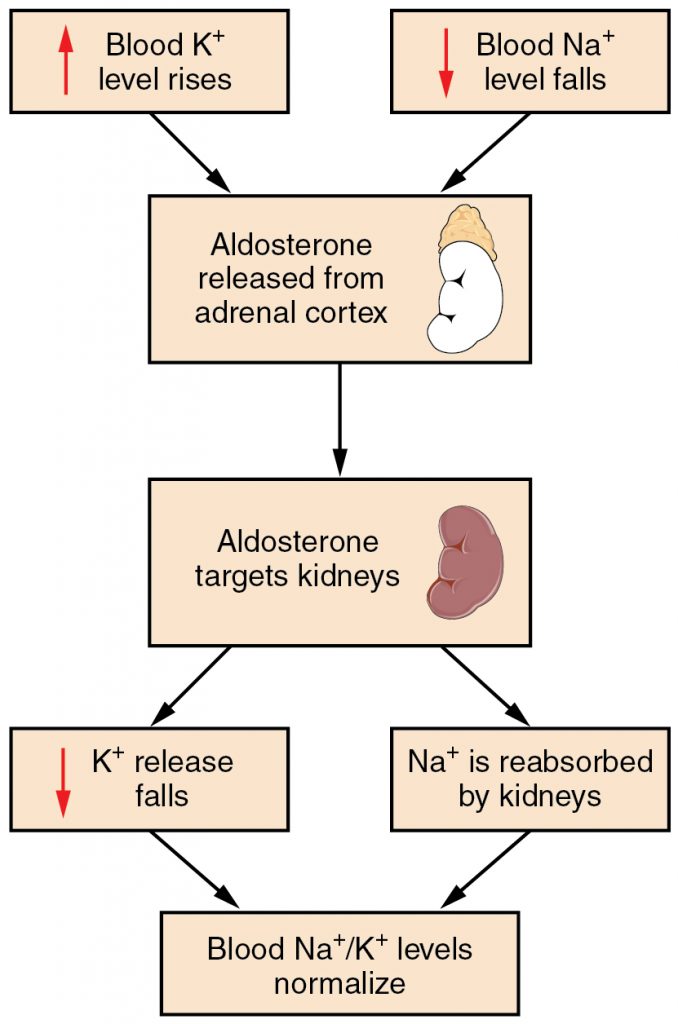
Potassium levels normally range from 3.5 to 5.1 mEq/L.[7] Refer to each agency’s normal reference range on the lab report. Potassium is the most abundant electrolyte in intracellular fluid and is maintained inside the cell by the sodium-potassium pump. Potassium is regulated by aldosterone in the kidneys and is obtained in the diet through consumption of foods such as bananas, oranges, and tomatoes. See Figure 15.13[8] for an illustration of potassium regulation by aldosterone. Recall that aldosterone causes reabsorption of sodium and excretion of potassium in the distal tubule of the kidneys. In response to potassium levels rising or sodium levels falling in the bloodstream, the adrenal cortex releases aldosterone and targets the kidneys. In response, the kidneys excrete potassium and reabsorb sodium. Potassium is also impacted by the hormone insulin that moves potassium into the cells from the ECF.[9]
Potassium is necessary for normal cardiac function, neural function, and muscle contractility, including effective contractility of the cardiac muscles. Abnormal potassium levels can cause significantly abnormal heart rhythms and contractility. Potassium is poorly conserved by the body and much is lost with urine output. For this reason, it is often necessary to provide potassium supplements when administering loop and thiazide diuretics because potassium is excreted from the kidneys along with water.[10] Potassium supplementation can be given orally or by IV infusion mixed with fluids. Potassium must NEVER be administered IV push because it can immediately stop the heart.
Hyperkalemia refers to increased potassium levels in the blood. Hyperkalemia can be caused by kidney failure, metabolic acidosis, and administration of potassium-sparing diuretics or oral/intravenous potassium supplements. Signs and symptoms of hyperkalemia are generally cardiac in nature and include irritability, cramping, diarrhea, and electrocardiogram (ECG) abnormalities. As hyperkalemia worsens, ECG abnormalities may progress to cardiac dysrhythmias and cardiac arrest.
Treatment for hyperkalemia depends on the severity of the hyperkalemia symptoms. For mild symptoms, decreased potassium intake in the diet is helpful. Adjustment to medications contributing to increased levels of potassium may be indicated. For severe symptoms, administration of sodium polystyrene sulfonate (Kayexalate) orally or rectally helps bind excess potassium so it is excreted through the GI tract. Insulin may be administered to push potassium into cells and decrease serum potassium levels. When administering an insulin infusion, it is important to monitor blood glucose levels closely, often hourly per agency policy. The patient often requires supplemental IV dextrose to prevent low blood sugar levels when insulin is used for potassium reduction. IV calcium gluconate may also be given to prevent excess potassium from affecting cardiac muscle. This is a temporary measure and wears off quickly but allows time for other treatments to take effect and lower potassium levels before cardiac arrest develops. For severe symptomatic hyperkalemia, temporary hemodialysis may also be used to quickly decrease potassium levels.[11]
Hypokalemia refers to decreased potassium level in the blood. Hypokalemia can be caused by excessive vomiting, diarrhea, potassium-wasting diuretics, and insulin use, as well as lack of potassium in the diet. Signs and symptoms of hypokalemia include weakness, arrhythmias, lethargy, and a thready pulse. View helpful mnemonics for hypokalemia using the following hyperlink. Treatment for hypokalemia includes increasing oral intake of potassium in the diet and oral or IV potassium in fluids supplementation. It is important to remember that administering IV potassium too quickly can cause cardiac arrest. In fact, potassium is one of the ingredients used during lethal injection to stop the heart.
View helpful mnemonics for hypokalemia at Hypokalemia NCLEX Review Notes.
Calcium
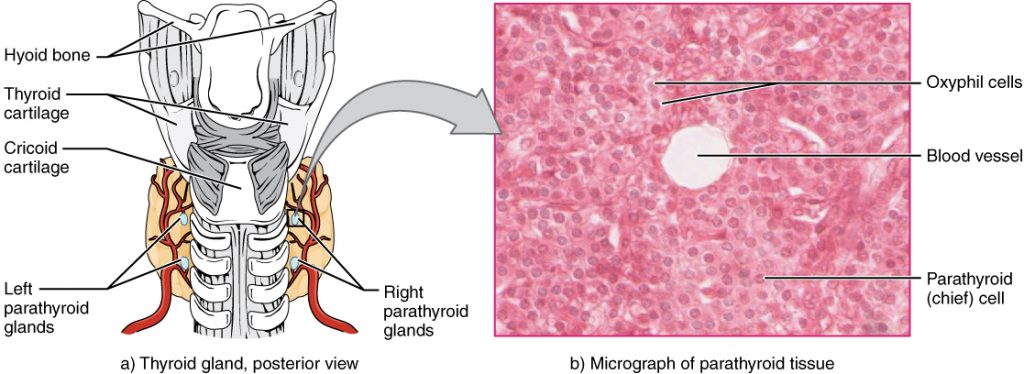
Calcium levels normally range from 8.6-10.2 mg/dL.[13] Refer to each agency’s normal reference range on the lab report. Calcium circulates in the bloodstream, but the majority is stored in bones. Calcium is important for bone and teeth structure, nerve transmission, and muscle contraction. Calcium excretion and reabsorption are regulated by the parathyroid hormone (PTH) that is secreted from the parathyroid glands near the thyroid. See Figure 15.14[14] for an illustration of the parathyroid glands. As PTH is secreted in response to low calcium levels in the blood, calcium is reabsorbed in both the kidneys and the intestine and released from the bones to increase serum calcium levels. Calcium is also affected by dietary intake and physical activity. Activity causes calcium to move into bones whereas immobility causes the release of calcium from bones, which cases them to become weak. Dietary sources of calcium include dairy products, green leafy vegetables, sardines, and whole grains.[15]
Hypercalcemia refers to an increased calcium level. It can be caused by prolonged immobilization that allows calcium to leach out of the bones and into the serum. Additionally, there are many types of cancers that may cause excessive calcium release from bones. Hypercalcemia can also be caused by hyperparathyroidism and parathyroid tumors, which can cause too much PTH secretion, causing too much calcium to be reabsorbed in the kidneys and intestines and released from bone.
Signs and symptoms of hypercalcemia often impact the gastrointestinal and musculoskeletal systems. These symptoms include nausea, vomiting, constipation, increased thirst and/or urination, and skeletal muscle weakness. Treatment for hypercalcemia includes decreasing calcium intake in the diet, phosphate supplementation (which has an inverse relationship to calcium), hemodialysis, surgical removal of the parathyroid gland (if hyperparathyroidism is causing the hypercalcemia), and weight-bearing exercises as tolerated.[16]
Hypocalcemia refers to a decreased calcium level in the blood. Hypocalcemia can be caused by hypoparathyroidism where not enough PTH is excreted, causing a decreased reabsorption of calcium and decreased release of calcium from the bones. Hypocalcemia is also caused by vitamin D deficiency and renal disease. Because phosphorus is inversely related to calcium, an abnormally high phosphorus level as seen with renal failure can also result in hypocalcemia.
Signs and symptoms of hypocalcemia often impact the musculoskeletal and nervous systems. These include paresthesias (numbness and tingling) of the lips, tongue, hands and feet, muscle cramps, and tetany. Chvostek’s sign is a classic sign of acute hypocalcemia and is an involuntary twitching of facial muscles when the facial nerve is tapped. A second classic sign of acute hypocalcemia is Trousseau’s sign where a hand spasm is caused by inflating a blood pressure cuff to a level above systolic pressure for 3 minutes. See a video of a patient experiencing Chvostek’s and Trousseau’s signs in the hyperlink below. Treatment of hypocalcemia includes increasing oral intake of dietary calcium and vitamin D and oral or IV calcium supplementation and decreasing the phosphorus level if it is elevated.[17]
View a video of a patient exhibiting Chvostek’s Sign and Trousseau’s Signs of hypocalcemia.
Phosphorus
Phosphorus levels typically range from 2.5-4.0 mg/dL. Refer to each agency’s normal reference range on the lab report. Phosphorus is stored in the bones and is predominantly found in the ICF with small amounts in the ECF. Phosphorus is important in energy metabolism, RNA and DNA formation, nerve function, muscle contraction, and for bone, teeth, and membrane building and repair. Phosphorus is excreted by the kidneys and absorbed by the intestines. Dietary phosphorus sources include dairy products, fruits, vegetables, meat, and cereal.[18]
Hyperphosphatemia refers to an increased phosphorus level in the blood and can be caused by kidney disease, crush injuries, or overuse of phosphate-containing enemas. Hyperphosphatemia itself is usually asymptomatic, but signs of associated hypocalcemia may be present due to the inverse relationship between phosphorus and calcium. Treatment for hyperphosphatemia includes decreasing intake of phosphorus, administration of phosphate-binder medications to help with excretion, and hemodialysis.[19]
Hypophosphatemia is a decreased phosphorus level in the blood. Acute hypophosphatemia can be caused by acute alcohol abuse, burns, diuretic use, respiratory alkalosis, resolving diabetic ketoacidosis, and starvation. Chronic hypophosphatemia is caused by hyperparathyroidism, vitamin D deficiency, prolonged use of phosphate binders, and hypomagnesemia or hypokalemia. Hypophosphatemia is usually asymptomatic, but in severe cases, it can cause muscle weakness, anorexia, encephalopathy, seizures, and death. Treatment for hypophosphatemia includes treating what is causing the imbalance, oral or IV phosphorus replacement, and increased phosphate-containing foods in the diet.[20]
Magnesium
Magnesium levels typically range from 1.5-2.4 mEq/L. Refer to each agency’s reference range on the lab report. Magnesium is essential for normal cardiac, nerve, muscle, and immune system functioning. About half of the body’s magnesium is stored in the bones. About 1% is stored in ECF and the rest is found in ICF.[21] Dietary sources of magnesium include green leafy vegetables, citrus, peanut butter, almonds, legumes, and chocolate.
Hypermagnesemia refers to an elevated magnesium level in the blood. It is usually the result of renal failure, excess magnesium replacement, or use of magnesium containing laxatives or antacids. Signs and symptoms of hypermagnesemia include bradycardia, weak and thready pulse, lethargy, tremors, hyporeflexia, muscle weakness, and cardiac arrest. Treatment for hypermagnesemia involves increasing fluid intake, discontinuing magnesium-containing medications, and in severe cases, hemodialysis or peritoneal dialysis. Additionally, administration of calcium gluconate can be helpful to reduce the cardiac effects of hypermagnesemia until the magnesium level can be lowered.[22]
Hypomagnesemia refers to decreased magnesium level in the blood. It typically results from inadequate magnesium in the diet, or from loop diuretics that excrete magnesium. Patients with alcohol use disorder often have hypomagnesemia due to concurrent poor diet and impaired nutrient absorption that occurs with alcohol consumption. Chronic proton pump inhibitor use can also cause hypomagnesemia due to impaired nutrient absorption.
Signs and symptoms of hypomagnesemia include nausea, vomiting, lethargy, weakness, leg cramps, tremor, dysrhythmias, and tetany that is associated with concurrent hypocalcemia that can occur with hypomagnesemia. Treatment for hypomagnesemia consists of increasing dietary intake of magnesium containing foods and oral or IV magnesium supplementation.[23]
See Table 15.4 for a comparison of causes, symptoms, and treatments of different electrolyte imbalances.
| Elevated Level | Decreased Level | |
|---|---|---|
| Sodium (Na+)
Normal range 136-145 mEq/L |
Hypernatremia
Causes: Excessive salt intake Symptoms: Lethargy, irritability, seizures, and weakness Treatments: Rehydrate w/ D5W and increase water intake |
Hyponatremia
Causes: Excessive water intake and diuretics Symptoms: Headache, confusion, coma Treatments: 3% NS and fluid restriction |
| Potassium (K+)
Normal range 3.5-5.1 mmol/L |
Hyperkalemia
Causes: Kidney dysfunction, excessive potassium intake, and ACE inhibitors Symptoms: Cardiac arrhythmias, cramping, diarrhea, and irritability Treatments: Limit potassium in diet, loop diuretic, insulin, dialysis, and kayexalate |
Hypokalemia
Causes: Loop and thiazide diuretics and IV administration of insulin Symptoms: Weakness, arrhythmias, lethargy, and thready pulse (WALT) Treatments: PO/IV potassium and increase K+ in diet |
| Calcium (Ca++)
Normal range 8.6 -10.2 mg/dL |
Hypercalcemia
Causes: Overactive parathyroid glands and cancer Symptoms: Nausea, vomiting, constipation, and thirst Treatments: Decrease calcium in diet, increase mobility, and administer phosphorous |
Hypocalcemia
Causes: Diuretic use and removal of parathyroid glands Symptoms: Numbness, tingling, Chvotek’s sign, and tetany Treatments: Increase Ca++ in diet and IV/PO calcium |
| Magnesium (Mg+)
Nornal range 1.5-2.4 mg/dL |
Hypermagnesemia
Causes: Kidney disease and excessive magnesium intake (i.e., laxatives and antacids) Symptoms: Muscle weakness, bradycardia, asystole, tremors, and slow reflexes Treatments: Dialysis, increased fluid intake, and stopping medications containing Mg+ |
Hypomagnesemia
Causes: Diuretics, undernutrition, and long-term alcohol use disorder
Treatments: Increase Mg+ in diet and PO/IV magnesium |
- Lab Tests Online. (2019). Sodium. https://labtestsonline.org/tests/sodium↵
- “thirsty-4294629_960_720.png” by Conmongt is licensed under CC0↵
- Lewis, J. L., III. (April 2020). Hypernatremia. Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/electrolyte-disorders/hypernatremia↵
- This work is a derivative of StatPearls by Brinkman, Dorius, and Sharma and is licensed under CC BY 4.0↵
- Lewis, J. L., III. (April 2020). Hyponatremia. Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/electrolyte-disorders/hyponatremia↵
- Forciea, B.(2017, April 24). Fluids and electrolytes sodium. [Video]. YouTube. All Rights Reserved. Video used with permission. https://youtu.be/ar-WrfC7SJs↵
- Lab Tests Online. (2019). Potassium. https://labtestsonline.org/tests/potassium↵
- “2711_Aldosterone_Feedback_Loop-01.jpg” by OpenStax College is licensed under CC BY 3.0. Access for free at https://openstax.org/books/anatomy-and-physiology/pages/26-3-electrolyte-balance↵
- This work is a derivative of Anatomy & Physiology by OpenStax and is licensed under CC BY 4.0. Access for free at https://openstax.org/books/anatomy-and-physiology/pages/1-introduction↵
- Lewis, J. L., III. (April 2020). Overview of disorders of potassium concentration. Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/electrolyte-disorders/overview-of-disorders-of-potassium-concentration↵
- Lewis, J. L., III. (April 2020). Hyperkalemia. Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/electrolyte-disorders/hyperkalemia↵
- Forciea, B. (2017, April 26). Fluids and electrolytes potassium. [Video]. YouTube. All rights reserved. Video used with permission. https://youtu.be/SNAiGaaYkvs↵
- Lab Tests Online (2017). Calcium. https://labtestsonline.org/tests/calcium↵
- "1814_The_Parathyroid_Glands.jpg” by OpenStax is licensed under CC BY 3.0. Access for free at https://openstax.org/books/anatomy-and-physiology/pages/17-5-the-parathyroid-glands↵
- Lewis, J. L., III. (April 2020). Overview of disorders of calcium concentration. Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/electrolyte-disorders/overview-of-disorders-of-calcium-concentration↵
- Lewis, J. L., III. (April 2020). Hypercalcemia. Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/electrolyte-disorders/hypercalcemia↵
- Lewis, J. L., III. (April 2020). Hypocalcemia. Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/electrolyte-disorders/hypocalcemia↵
- Lewis, J. L., III. (April 2020). Overview of disorders of phosphate concentration. Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/electrolyte-disorders/overview-of-disorders-of-phosphate-concentration↵
- Lewis, J. L., III. (April 2020). Hyperphosphatemia. Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/electrolyte-disorders/hyperphosphatemia↵
- Lewis, J. L., III. (April 2020). Hypophosphatemia. Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/electrolyte-disorders/hypophosphatemia↵
- Lewis, J. L., III. (April 2020). Overview of disorders of magnesium concentration. Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/electrolyte-disorders/overview-of-disorders-of-magnesium-concentration↵
- Lewis, J. L., III. (April 2020). Hypermagnesemia. Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/electrolyte-disorders/hypermagnesemia↵
- Lewis, J. L., III. (April 2020). Hypomagnesemia. Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/electrolyte-disorders/hypomagnesemia 7/7/20 ↵


