1.44: Surgery for Pharyngeal Pouch / Zenker's Diverticulum
- Page ID
- 17685
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)OPEN ACCESS ATLAS OF OTOLARYNGOLOGY, HEAD & NECK OPERATIVE SURGERY
SURGERY FOR PHARYNGEAL POUCH / ZENKER’S DIVERTICULUM
Daniel Schuster, James Netterville, Johan Fagan
This chapter provides an overview of management of Zenker’s divertula (ZD), including detailed surgical technique, with an emphasis on effective therapy and management.
Zenker, a German pathologist, first reported 27 hypopharyngeal pulsion diverticula in 1878.1 In 1907, Killian, described the triangular dehiscence between the cricopharyngeus and inferior constrictor muscles that is the anatomic site of formation of Zenker’s Diverticula.2 (Figure 1)
Although there is a role for open surgery, management has evolved to effective, safe endoscopic techniques with proven low morbidity.
Etiology
The etiology has been debated for more than a century. Historically, authors have theorized that trauma, congenital upper esophageal strictures, thyroid goiter, or foreign bodies may play role.3 Gastroesophageal reflux, cricopharyngeal achalasia, persistently elevated resting tone of the cricopharyngeus, structural abnormality of the cricopharyngeus muscle, and central or peripheral neurologic disease have also been proposed as contributing or causal factors, though convincing evidence is lacking.3
However, it was Zenker who correctly described it as a pulsion diverticulum and put earlier theories to rest.1 Since that time most authors agree that anatomic predisposition plays a central role in the pathogenesis, though the exact aetiology is still a subject of debate.
It is now widely accepted that dysfunction of the cricopharyngeus muscle plays a central aetiological role. High intraluminal pressures at the level of Killian’s triangle result in herniation of esophageal mucosa and submucosa through this area of inherent muscular weakness. Some advocate that high intraluminal pressures produced by incoordination of the inferior constricttor muscle against a closed cricopharyngeus is responsible, while others state that high pressures are a result of the anatomic relationship between the relatively wide hypopharynx and a narrower esophageal inlet through which food boluses pass.5 Killian’s triangle itself is of variable size, and patients with larger areas of inherent muscular weakness are likely more predisposed to a ZD.
Pathophysiology
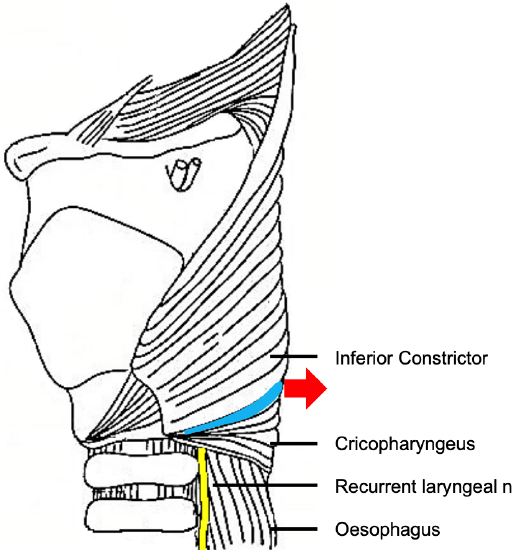
Figure 1: Red arrow shows where ZD extrudes through Killian’s dehiscence (blue) between the inferior constrictor and the cricopharyngeus muscles
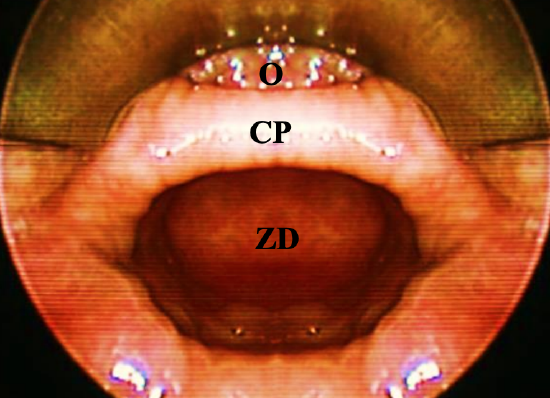
Figure 2: Cricopharyngeal bar (CP) containing the cricopharyngeus muscle, which separates esophagus (O) from ZD
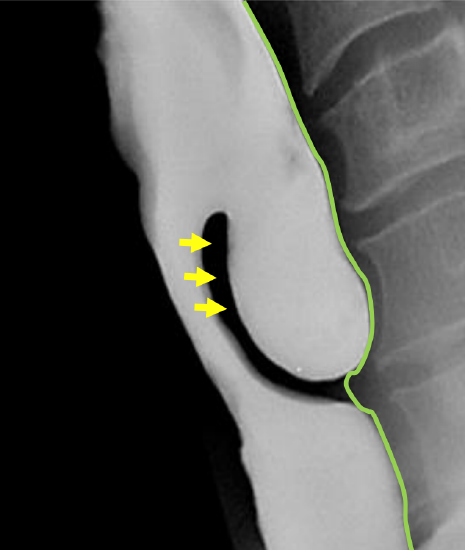
Figure 3: Barium swallow of ZD. Note location of cricopharyngeus muscle (arrows) in upper part of party wall between esophagus and ZD; and how buccopharyngeal fascia contains the diverticulum (green line)
ZD is a pulsion diverticulum or herniation of esophageal mucosa and submucosa through Killian’s triangle, which is immediately proximal to the cricopharyngeus muscle (Figures 1, 2, 3).
Of surgical importance is that the entire diverticulum is contained within the buccopharyngeal fascia, which itself is not involved in the formation of the diverticulum (Figure 3). The buccopharyngeal fascia is in effect displaced posteriorly by the posterior wall of the diverticulum, creating a safer plane to divide the “party wall” (that contains the cricopharyngeus) without penetrating the fascia and entering the prevertebral space (Figure 3). This anatomic relationship between the ZD and the surrounding buccopharyngeal fascial layer is the key to understanding how the upper digestive tract remains separated from the retropharyngeal space when incising the anterior wall of the diverticulum, or in the case of an isolated endoscopic cricopharyngeal myotomy. Disrupting this fascial layer could theoretically increase the likelihood of developing mediastinitus.
However, when endoscopically dividing a hypertrophic cricopharyngeus muscle in the absence of a ZD, the buccopharyngeal fascia is situated immediately behind the cricopharyngeus muscle, and great care has to be taken to preserve this fascial layer when completely dividing the cricopharyngeus muscle. Despite initial fears that this fascia could not be preserved during endoscopic cricopharyngeal myotomy, Chang et al demonstrated in a cadaveric study that the buccopharyngeal fascial layer remained histologically intact with carbon dioxide (CO2) laser cricopharyngeal myotomy.4
Clinical Presentation
ZD occurs predominantly in the elderly; it is rarely seen in patients <40 years of age. A thorough history may raise suspicion of a ZD. Hallmark symptoms are progressive dysphagia coupled with postprandial regurgitation of undigested food. When associated with halitosis, weight loss, noisy deglutition, coughing, globus sensation, and recurrent unprovoked aspiration, the history alone is pathognomonic.5 Symptoms may exist for weeks to years, and the severity of the symptoms generally correlates with the size of the ZD. Physical examination is often normal, although occasionally pooling of saliva in the hypopharynx may be noted on laryngoscopy that may or may not clear with swallowing. A neck mass is rarely palpable. Rarely, Boyce’s sign, or a swelling in the neck that gurgles with palpation, is present.6
Clinicians must mindful that, although rare (incidence <0.5%), carcinoma can occur in a ZD7. Blood-tinged regurgitated food should raise suspicion for this, and is an indication that observation is not a reasonable option. Barium swallow frequently shows filling defects within the sac which usually represent undigested food. However, a constant filling defect on serial studies should raise concern of a tumor.5
Special Investigations
Diagnosis is confirmed with a contrast swallow and videofluoroscopy (Figure 3). Laryngoscopy is done to rule out other causes of dysphagia, as well as to document vocal cord dysfunction. Preoperative evaluation must include assessment of the function of the lower esophageal sphincter, as cricopharyngeal myotomy in patients with an incompetent lower esophageal sphincter places them at risk of developing severe gastroesophageal and laryngopharyngeal reflux.
Surgical Anatomy
A ZD is a thin-walled sac lined by stratified squamous epithelium with a thin layer of fibrous submucosa. The muscular layer of the hypopharynx is absent from its wall. The posterior mucosal herniation passes through Killian’s dehiscence/ triangle and is located between the inferior fibers of the inferior pharyngeal constrictor and the superior edge of cricopharyngeus muscle (Figures 1, 3). The common (party) wall contains the cricopharyngeus at its upper edge and separates the esophagus from diverticulum (Figures 2, 3).
It is important to understand that after the ZD herniates through Killian’s dehiscence, it expands posterior to the cricopharyngeus and the superior esophageal muscles layers but is still contained within the perioesophageal fascia. This protective layer of investing facia surrounding both the esophagus and ZD is the key to endoscopic division of the cricopharyngeus muscle and the entire party wall between the ZD and the esophagus. Without this fascial layer, saliva would be likely to track into the mediastinum causing mediastinitis.
Treatment
Observation with dietary manipulation is a reasonable option for a relatively small ZD, or in patients with minimal symptoms, or who pose a significant anesthetic risk.
Surgical approaches include the following:
- Endoscopic
- Cricopharyngeal myotomy
- Diverticulotomy
- Stapling
- Laser
- Electrocoagulation
- External transcervical
- Cricopharyngeal myotomy
- Inversion
- Diverticulopexy (suspension)
- Diverticulectomy
- Suturing
- Stapling
Most authors currently advocate endoscopic surgery as the preferred treatment, and many studies have reported endoscopic techniques to be both safe and effective.8-12 Advantages compared to external procedures are brief anesthetic time, early oral intake, short hospitalization, minimal pain and that revision surgery, either endoscopic or external, is not compromised by scarring.
As with any surgery, the risks and benefits of surgery must be carefully weighed, particularly because ZD patients are generally elderly and often infirm.
Endoscopic procedures
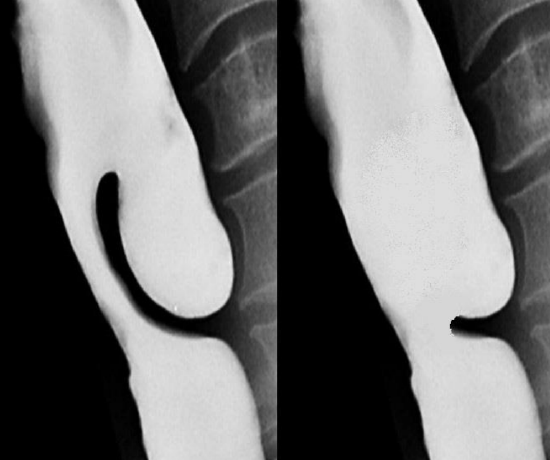
Figure 4: Before and after endoscopic diverticolotomy with a stapler: Cricopharyngeus and the party wall between the ZD and the esophagus have been divided, incorporating the pouch in the esophagus. Note how the stapler is unable to divide the distal party wall resulting in a residual bar
Endoscopic surgery completely divides the common wall between the esophagus and the diverticulum (diverticulotomy), thereby incorporating the diverticulum into the esophageal lumen; and in the process also performs a cricopharyngeal myotomy (Figure 4).
Much debate exists regarding the most effective technique. The authors are in agreement with a study that found endoscopic CO2 laser to be more effective and to result in significantly less recurrence than endoscopic stapling.10 This may be because the endoscopic stapler does not divide the most distal part of the common wall between the esophagus and the diverticulum (Figure 4). In parts of the world where neither a stapler nor CO2 laser is available, a long suction cautery will work in the place of the laser.
Consideration must be given to flexibility and disease of the cervical spine. Communication with the anesthesia care team should include a discussion regarding the need for paralysis as a component of the general anesthetic, as muscular tone may limit visualization and complicate placement of the scope.
The choice of operating diverticuloscope depends on the procedure to be performed.

Figure 5a: Benjamin diverticuloscope

Figure 5b: Weerda bivalve diverticuloscope
Benjamin diverticuloscope: (Figure 5a) For CO2 laser diverticulotomy, the authors have found the Benjamin diverticuloscope (Storz) to provide the optimum combination of visualisation and ease of placement. Because it has straightened tips, rather than outward-curved tips of the Dohlman scope that was first used for endoscopic surgery, it is often able to be placed in patients in whom placement of the original Dohlman scope would have been difficult.
Weerda diverticuloscope: The long bivalved Weerda diverticuloscope (Storz) provides additional anterior-to-posterior volume by opening the blades of the scope; this is essential for using the stapler technique. However, the width of the proximal end of the scope makes it more difficult to insert in patients with narrow interdental width.
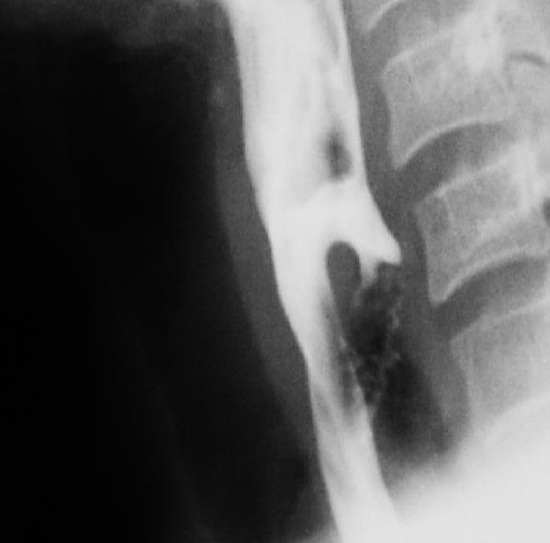
Figure 6: Perforated pouch
Passing a diverticuloscope can be difficult or impossible in some patients. It is therefore important to caution patients that an endoscopic approach may have to be abandoned due to poor access. In less experienced hands the scope is a particularly dangerous instrument as it may be difficult to insert and may penetrate the posterior pharyngeal wall or the pouch causing cervical sepsis and mediastinitis (Figure 6). It is therefore crucial that the surgeon exercises sound judgement and abandons the procedure before causing an iatrogenic perforation.
Surgical steps for endoscopic diverticulotomy
- Administer broad spectrum antibiotics perioperatively
- General anesthesia is done with orotracheal intubation (nasotracheal intobation hampers access)
- Protect the upper teeth with a gum guard
- Study the barium swallow to determine the dimensions of the ZD and the locations of the neck of the ZD and of the esophageal inlet
- Commence endoscopy with an anterior commissure or Dedo laryngoscope
- Pass via the right pyriform sinus into the postcricoid region
- Examine the diverticulum
- Identify the esophageal lumen
- Suction retained food and secretions from the ZD
- Use 0-degree Hopkins rod to closely examine the area (including the hypopharynx) – note any abnormalities that could contribute to dysphagia
- Pass a blunt probe into the esophageal lumen and press it downwards to stretch the cricopharyngeus muscle; relaxation of the cricopharyngeus muscle permits easier passage of the anterior lip of the Benjamim or Weerda scopes into the esophagus
- If the lumen of the esophagus is difficult to cannulate with the laryngoscope
- Insert a rubber catheter, a suction catheter, or a long 4 mm round anesthesia lumen finder into the lumen of the esophagus
- Then pass the diverticuloscope over the catheter or lumen finder which will pull down on the esophageal lumen and direct the anterior lip of the scope into the esophagus
- Inspect the ZD for malignancy
- Carefully remove the laryngoscope
- Perform rigid esophagoscopy
- Inspect the esophagus
- Dilating the esophageal opening with the scope facilitates passage of diverticuloscope
- Use of Benjamin diverticuloscope to engage the ZD (Figure 5a)
- It is not always possible to insert the scope due to anatomical limitations
- Lubricate the scope with surgical jelly
- Extend the neck posteriorly as far as the cervical spine allows
- Pass the scope into the right pyriform sinus gently lifting the tip of the scope as it is directed medially under the postcricoid region
- Advance the scope with the anterior limb passing into the esophageal lumen
- The scope is often stable with no need for suspension
- Use of Weerda diverticuloscope to engage the ZD (Figure 5b)
- Pass the closed end of the scope into the pouch taking care not to perforate the thin wall of the pouch
- Open the blades, and retract the scope until the esophageal opening appears anteriorly
- Pass the anterior blade of the scope into the esophagus and keep the posterior blade in the pouch
- Distract the blades further to bring the party wall into view (Figure 1)
- Suspend the scope with a scope holder
- Clear the pouch of food debris
- Proceed to cricopharyngeal myotomy +/- diverticulotomy
Endoscopic CO2 laser cricopharyngeal myotomy +/- diverticulotomy
CO2 laser has the advantage of dividing the party wall up to the inferior aspect of the pouch. When a stapler is used there is always a residual inferior lip of the pouch that cannot be divided by the device due to its design; the laser is often used to divide this residual bar. In the era of critical cost containment, the laser therefore has the advantage of being able to complete the entire task, whereas the stapler may need assistance of further division of the inferior party wall. Asymptomatic postsurgical subcutaneous emphysema occurs slightly more frequently following CO2 laser than with the endoscopic stapler.
- Set the CO2 laser at 5-10 W, CW mode and with a slightly defocused spot size to improve hemostasis
- Visualize the common party wall using an operating microscope with an integrated CO2 laser microsled
- Diverticulotomy
- Use the laser to transect the mucosa and cricopharyngeus muscle in the in the midline of the common wall
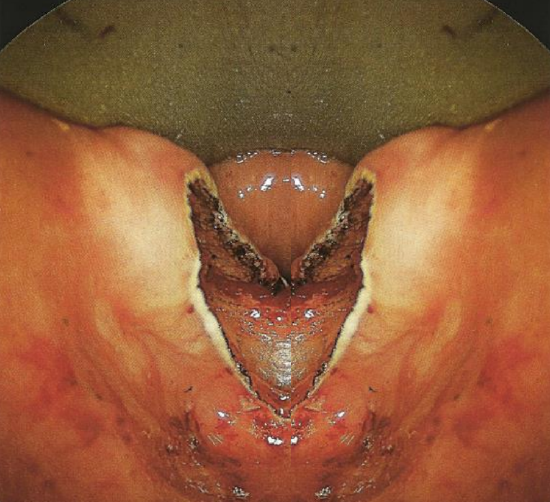
Figure 7: Party wall has been divided. Careful inspection demonstrates that all layers of the cricopharyngeous muscle and mucosa have been divided to inferior aspect of sac
- Continue to transect the party wall in the midline with the laser down to its most inferior edge (Figure 7)
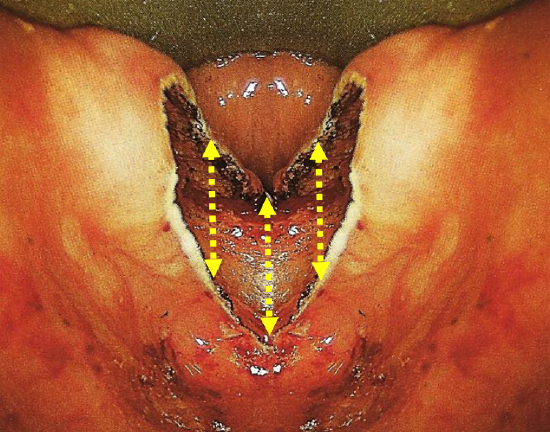
Figure 8: Position of 3 mucosal sutures
- Though not required (because of the intact perioesophageal fascia as previously discussed (Figure 3), we place three sutures to close the mucosal defect (Figure 8)
- The first suture closes the superior esophageal mucosal margin to the inferior edge of the posterior wall of the diverticulum in the midline
- Then two additional sutures are placed one on each side of the midline to approximate the mucosal edges
- The added length of the 4-0 RB1 taper needle, 27-inch Vicryl suture facilitates endoscopic tying technique needed to tie the sutures down through the scope
- Inspect the area for hemostasis
- Remove the diverticuloscope
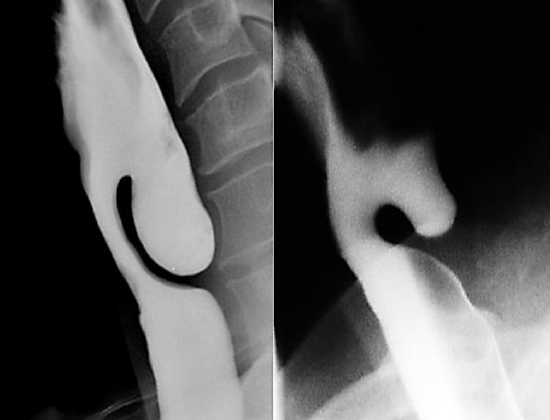
Figure 9: ZD on right side would be very difficult to treat with the Stapler and is best done with CO2 laser, as it is too shallow for the stapling technique
CO2 laser is particularly useful for pouches of <4 cm because the stapler does not divide the distal 1-2 cm of the party wall (Figure 9).
Cricopharyngeal myotomy
Cricopharyngeal myotomy forms part of the diverticulotomy procedure as described above, but may also be done in isolation for very small ZDs or for cricopharyngeal spasm
- Insert a diverticuloscope with its two tips anterior and posterior to the cricopharyngeus muscle
- Identify the prominence of the cricopharyngeus muscle
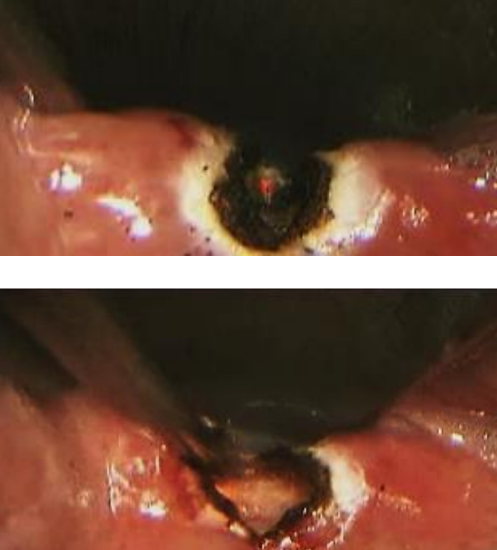
Figure 10: Transmucosal laser cricopharyngeal myotomy: incising muscle (top); and completed myotomy (below)
- Use the laser to transect the mucosa overlying the muscle in the midline (Figure 10)
- Proceed to meticulously completely transect the cricopharyngeus muscle without disrupting the posterior layer of perioesophageal fascia (Figure 10)
Staple-assisted endoscopic diverticulotomy
Endoscopic stapling, though not our preferred method, is a valuable method to use if CO2 laser is not available.
Compared to laser or diathermy, it is quicker (few minutes), has a lesser risk of perforation and mediastinitis, provides good hemostasis, and avoids thermal injury to the recurrent laryngeal nerve. The principal disadvantage is that it does not divide the distal 1-2 cm of the wall of the diverticulum and is therefore not suited to small pouches (Figure 9).
The first few procedural steps are identical to the CO2 laser approach described above. The procedures only differ following inspection and stretching of the cricopharyngeus muscle.
- Insert a bivalve (Storz) diverticuloscope; this scope is required to provide a wide enough exposure to introduce a stapler

Figure 11: Example of disposable linear stapler/cutter with ends open and closed
- Introduce the endoscopic stapler (Figure 11)
- A long 0-degree Hopkins rod can be used to assist visualization
- Place the two ends of the stapler astride the party wall in the midline, with one end in the esophagus, and the other in the pouch
- The 3rd author places the anvil (not the cartridge) of the stapler into the pouch, because it allows one to divide a greater length of the party wall; others prefer to place the anvil in the esophagus due to its smaller diameter9
- Advance the stapler to the fundus of the diverticulum or as far as it will go
- Fire the stapler
- Firmly squeeze the handle that approximates the anvil and cartridge
- Activate the cutting blade with the handle or slide
- Release the stapler and remove the instrument
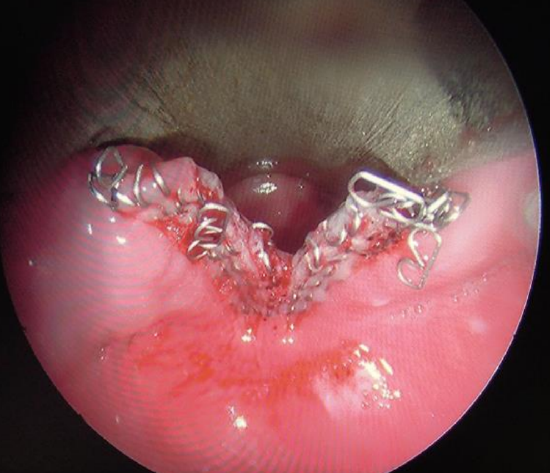
Figure 12: Diverticulotomy with stapler
- Examine the field using the Hopkins rod to determine whether an additional cartridge should be used to divide any remaining party wall (Figure 12)
- Inspect the diverticulum to ensure it has not been perforated
- Remove the instrumentation

Figure 13: Note distance between tip of cartridge, and staples and cutting groove
It is important to note that the tip of the stapler cartridge extends beyond the end of the staples, and that the staples extend beyond the razor incision (Figure 13). The result is that this method will always leave some residual sac, which we believe contributes to its higher recurrence rate.
One option to minimize this problem is to place traction sutures on either side of the planned myotomy prior to firing the stapler. Pulling on these sutures during stapler activation maximizes the length of the razor cut. Some evidence exists that this may increase the long-term success of this technique.13
Endoscopic diverticulotomy with electrocautery
In resource-limited settings, access to endoscopic staplers and CO2 laser may not be possible due to the significant associated costs. Consequently, we believe that another option for endoscopic treatment of ZD without the expense of this equipment is to use electrocautery to perform diverticulotomy, as was originally described by Dohlman.14 With this technique one exposes the common wall using a diverticuloscope in the same way as with the previous two procedures. A pair of insulated surgical forceps is then used to grasp the common party wall in the midline, and monopolar electrocautery is applied to the forceps. This is repeated as many times as is necessary to carry the myotomy down to the most inferior aspect of the common wall. Again, sutures may be placed on either side of the myotomy to help close the mucosa, though this is not necessary because the buccopharyngeal fascia, the key layer to maintaining separation between the digestive tract and the mediastinum, remains intact.
Postoperative care following endoscopic diverticulotomy
- Observe patients overnight (regardless of surgical technique)
- Monitor for signs and symptoms that signal mediastinitis, as early intervenetion to treat this rare but potentially fatal complication is mandatory; radiating back or chest pain, fever, and tachycardia despite adequate pain control should raise suspicion of mediastinitis
- Following CO2 laser or cautery diverticulotomy a small number of patients develop some subcutaneous emphysema; in our experience this almost never results in mediastinitis
- Start a clear liquid diet the morning following surgery
- Discharge the patient if he/she tolerates a clear liquid diet without concerning signs or symptoms
- The patient should remain on liquids and soft foods for several weeks to prevent esophageal rupture being caused by large solid food boluses
- Progress to a regular diet over a few weeks
Open Transcervical Diverticulectomy
The primary indication to treat ZD in an open fashion is inability to gain adequate exposure using transoral endoscopic techniques, or with an extensive, large ZD. Although rare, the anatomy of some patients will prevent adequate endoscopic exposure. As a result, all surgeons who treat ZD endoscopically should be familiar with open transcervical techniques and should be prepared to perform it if needed. Historically, different open procedures were performed including diverticulopexy and inversion if the pouch. In our experience, success rates are significantly higher with open diverticulectomy.
Surgical steps of Open Diverticulectomy
- Review the position of the diverticulum on the preoperative barium swallow
- Perform direct laryngoscopy with an anterior commissure laryngoscope
- Suction all food debris from the diverticulum
- Pack the diverticulum with vaseline gauze to assist with its identification
- Transorally insert a Maloney dilator into the esophagus; this helps identify relevant structures in the neck during the open dissection
- Make a 5-8 cm transverse cervical skin incision to the left of the midline at the level of the cricoid (Although rarely a large diverticulum will extend to the right of midline, it can still be delivered to the left side with the larynx rotated)
- Incise the platysma muscle
- Elevate subplatysmal flaps
- Dissect between the strap muscles, trachea, and thyroid gland medially, and the sternocleidomastoid, and the carotid/internal jugular vein vascular bundle laterally
- The superior laryngeal nerve defines the superior boundary of the dissection
- Palpate and identify the fullness of the cricothyroid joint
- It is now safe to place a double pronged skin hook under the posterior edge of the thyroid cartilage superior to this joint to rotate the entire laryngo-tracheal complex to present the ZD into the operative field
- Replace the skin hook with a Weitlaner self-retaining retractor to hold the operative field open
- With this laryngotracheal rotation the RLN is safe as long as one dissects in the posterior midline of the cricopharyngeus muscle
- At this point the ZD is not well defined, but rather a fullness that can be palpated deep to the peripharyngeal fascia
- Below this identify the firm dilator within the esophagus
- Dissect through the perioesophageal fascia until the ZD is isolated
- Bluntly dissect and elevate the ZD off the cricopharyngeus muscle
- The cricopharyngeus muscle can easily be palpated and visualized, stretched over the esophageal dilator
- Dissect with a blunt right angle dissector between the cricopharyngeus muscle and the underlying esophageal mucosa
- Divide the muscle layer from below the cricopharyngeus muscle up to the inferior border of the neck of the ZD
- One can also (with loupe magnification) use a 15 knife blade to divide the muscle layer down to the esophageal mucosa
- Use a suture to loosely tack the cricopharyngeus muscle end up to the region of the posterior border of the thyroid cartilage
- (One can at this point use a stapler to seal and divide the neck of the diverticulum; however, this adds significant expense to the procedure)
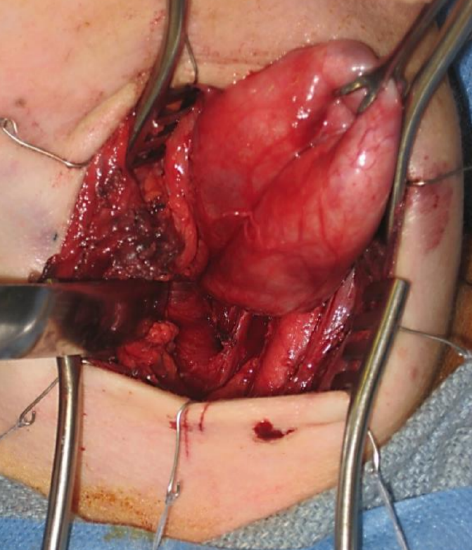
Figure 14: Left neck: The diverticular sac has been exposed and the overlying fascia is dissected off; note carotid artery lateral to the sac
- Open the diverticulum and inspect the mucosal surfaces (Figure 14)
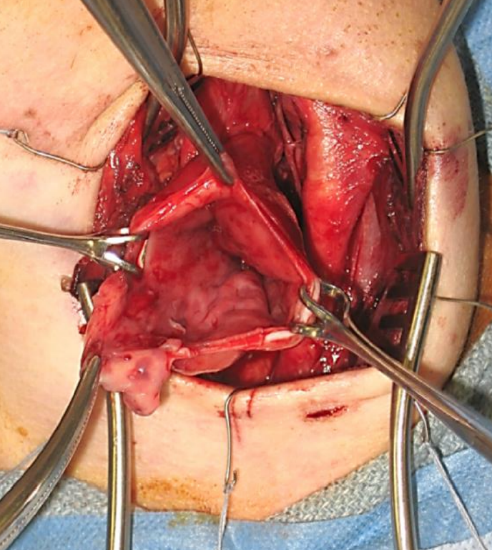
Figure 15: Left neck: The diverticular sac has been opened. Inspect the mucosal surfaces prior to doing a conservative resection
- Perform a conservative resection of the diverticulum, leaving about 1-2 cm of mucosa (Figure 15)
- Close the diverticulectomy defect using absorbable inverting mattress sutures transversely from superior-to-inferior (Do not close the defect vertically from side-to-side as this effectively narrows the esophageal lumen and predisposes to stricture formation)
- Irrigate the neck copiously
- Ensure hemostasis
- Insert a drain
- Close the neck in a layered fashion
- Insert a nasogastric feeding tube
- Continue with antibiotics for 24 hours
- Continue nasogastric tube feeding for about a week
- Introduce oral feeding if the patient is swallowing their saliva well with no symptoms at the end of the week
- If the patient’s neck is sore or they are having a hard time to swallow, confirm there is no leak on barium swallow before commencing oral feeding
Complications of surgery
Like all surgical procedures, operations performed for ZD are not without complications. When performing endoscopic procedures, care should be taken to avoid causing damage to the lips, teeth, tongue, and gums especially in cases of difficult exposure. Bleeding is a potentially dangerous complication, as a hematoma in the neck can result in airway compression. As a result, particular attention should be paid to achieving hemostasis during open procedures.
Perhaps the most feared complication of ZD surgery, which can occur in both endoscopic and open procedures, is esophageal perforation and a subsequent leak. Because the fascial planes in the neck are continuous with the mediastinum, leakage of esophageal contents into the neck can cause mediastinitis, which can be lethal if not quickly recognized and managed appropriately. In endoscopic procedures, perforation can occur in two main ways. The pouch itself can be perforated by advancing equipment too aggressively. This can also be caused by a stapling device, or an aggressively inserted suction or blunt probe. Alternatively, division of the common wall between the diverticulum and the esophagus can result in perforation if the dissection is carried too deeply behind the divided muscle layer. For a leak to occur, the perioesophageal fascia must be violated. Perforation and mediastinitis should be diagnosed promptly. Early signs include tachycardia, fever, and chest and neck pain. Crepitus may also occur. Any concern about this complication should prompt an immediate workup and empiric therapy. The patient should take nothing by mouth, and broad-spectrum antibiotics should be started empirically. Serial chest X-rays may demonstrate expanding subcutaneous air. A contrast swallow should be performed to establish if a clinical fluid leak is present through the perioesophageal fascia. If diagnosed early, this can usually be managed conservatively. A nasogastric tube for feeding may be necessary until the perforation heals.
Open procedures can result in additional complications. Injury to critical structures in the neck are the primary concern. The recurrent laryngeal nerve is of particular interest and can be identified if needed if the neck of the diverticulum is more inferior than is usual. If the dissection and ligation of the ZD is performed in the midline posteriorly the nerve is naturally avoided during this dissection.
Postoperative wound infection can occur as well, particularly if diverticular contents are spilled into the neck. This underscores the need to suction the sac endoscopically prior to opening the neck.
Excising too much of the sac wall with an open diverticulectomy procedure may cause a stricture.
Recurrence
Regardless of the technique used to treat ZD, perhaps the most common problem is recurrence. In our experience, this is more likely to occur after endoscopic stapling; we feel this is due to the inability to extend the diverticulotomy to the most inferior edge of the sac. In some cases, symptommatic recurrence can progress to the point that a revision procedure is indicated. When recurrence does occur, the diverticulum is often wider. In order to successfully address recurrence, the surgeon must extend the diverticulotomy all the way to the bottom of the sac and eliminate the shelf.
References
- Zenker, FA, von Ziemssen H. Dilatations of the esophagus. Cyclopedia Pract Med 1878; 3:46-8
- Killian G. La boudre de l’oesophage. Ann Mal Orelle Larynx 1907; 34:1
- Scher RL. Zenker’s Diverticulum. Cummings Otolaryngology-Head & Neck Surgery, 5th ed. 2010;74:986-97
- Chang CWD, Liou SS, Netterville JL. Anatomic Study of Laser-Assisted Endoscopic Cricopharyngeus Myotomy. Ann Otol Rhinol Laryngol 2005;114(12):897- 901
- van Overbeek JJM. Pathogenesis and methods of treatment of Zenker’s Diverticulum. Ann Otol Rhinol Laryngol 2003;112(7):583-93
- Siddiq M, Sood S, Strachan DR. Pharyngeal Pouch (Zenker’s diverticulum) Postgrad Med J 2001; 77:506-11
- Payne WS. The treatment of pharyngoesophageal diverticulum: the simple and complex. Hepatogastroenterology 1992; 39:109-14
- Smith SR, Genden EM, and Urken ML. Endoscopic stapling technique for the treatment of Zenker diverticulum vs standard open-neck technique: A direct comparison and charge analysis. Arch Otolaryngol Head Neck Surg 2002;128: 141-4
- Richtsmeier WJ. Endoscopic management of Zenker diverticulum: the staple-assisted approach. Am J Med 2003; 115(3A):175S178S
- Adam SI, Paskhover B, Sasaki CT. Laser versus stapler: outcomes in endoscopic repair of Zenker diverticulum. Laryngoscope 2012; 122:1961-6
- Maune S. Carbon dioxide laser diverticulostomy: a new treatment for Zenker diverticulum. Am J Med 2003;115-(3A): 172S174S
- Miller FR, Bartley J, Otto RA. The endoscopic management of Zenker diverticulum: CO2 laser versus endoscopic stapling. Laryngoscope 2006; 116:1608-11
- Bonavina L, Rottoli M, Bona D, Siboni S, Russo IS, Bernardi D. Transoral stapling for Zenker diverticulum: effect of the traction suture-assisted technique on long term outcomes. Surg Endosc; published online 27 April 2012
- Dohlman G. Endoscopic operations for hypopharyngeal diverticula. Proceedings of the Fourth International Congress of Otolaryngology, London, 1949:715-7
Suggested reading in Open Access Atlas
Authors
Daniel Schuster, M.D.
Dept. of Otolaryngology - Head and Neck Surgery
Vanderbilt University Medical Center
Nashville, TN, USA
daniel.schuster@vanderbilt.edu
James L. Netterville, MD
Mark C. Smith Professor and Director of Head and Neck Oncologic Surgery
Dept. of Otolaryngology – Head and Neck Surgery
Vanderbilt Bill Wilkerson Center
Nashville, TN, USA
james.netterville@vanderbilt.edu
Author & Editor
Johan Fagan MBChB, FCS(ORL), MMed
Professor and Chairman
Division of Otolaryngology
University of Cape Town
Cape Town, South Africa
johannes.fagan@uct.ac.za


