9.3: Vitamin C
- Page ID
- 40977
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Vitamin C is well-known for being a water-soluble antioxidant. Humans are one of the few mammals that do not synthesize vitamin C, making it an essential micronutrient. Other mammals that do not synthesize vitamin C include primates, guinea pigs, and other less prevalent species1. This means that some species like rats, mice, dogs and cats are not great options for human vitamin C research since it is not an essential nutrient for them.
Query \(\PageIndex{1}\)
Vitamin C's scientific names are ascorbic acid or ascorbate and the oxidized form is dehydroascorbic acid or dehydroascorbate. The structure of vitamin C is shown below.
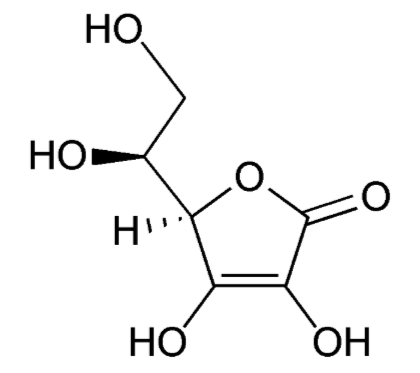
When ascorbic acid is oxidized, it forms semidehydroascorbate (1 degree of oxidation) and then dehydroascorbate (2 degrees of oxidation). The structure of dehydroascorbic acid is shown below.
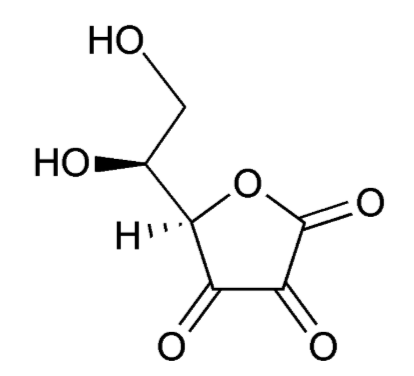
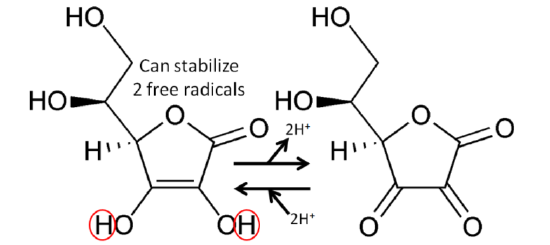
The figure below shows the reaction through which ascorbic acid can stabilize or quench 2 free radicals. The 2 circled hydrogens are lost and replaced by double bonds when ascorbic acid is oxidized to dehydroascorbic acid. Reducing dehydroascorbic acid back to ascorbic acid is the opposite reaction.
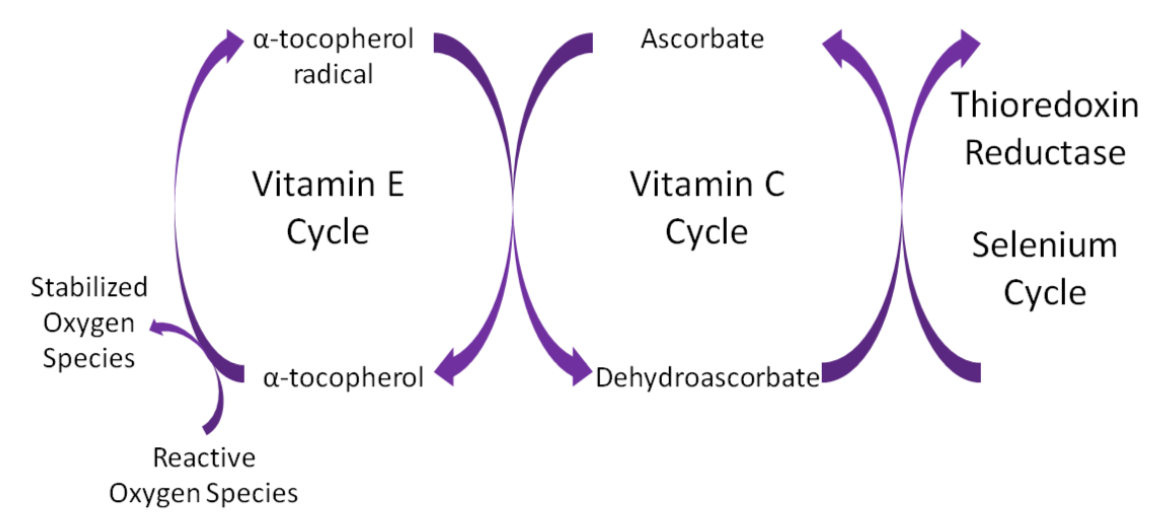
Ascorbic acid is believed to be a part of an antioxidant network (shown below) where it is oxidized to reduce alpha-tocopherol radicals. Dehydroascorbic acid can be reduced by thioredoxin reductase, a selenoenzyme, to regenerate ascorbic acid.
Query \(\PageIndex{2}\)
Vitamin C Absorption & Tissue Accumulation
Vitamin C is found in foods primarily as ascorbic acid (80-90%), but dehydroascorbic acid (10-20%) is also present. The bioavailability of vitamin C is high at lower doses as shown below, but drops to less than 50% at higher doses.
| Dose (mg) | % Bioavailability |
|---|---|
| 200 | 112 |
| 500 | 73 |
| 1250 | 49 |
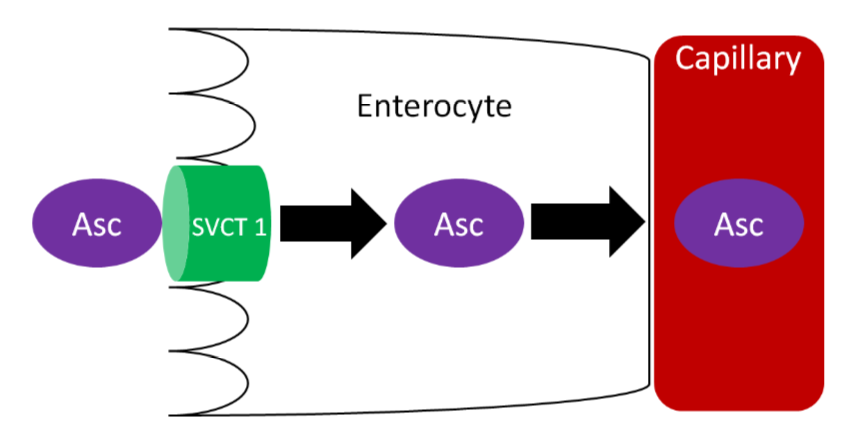
Ascorbic acid is actively absorbed by the sodium vitamin C cotransporter (SVCT) 1. This active transport is driven by the sodium electrochemical gradient created by sodium-potassium ATPase. Ascorbic acid then diffuses into the capillary and ultimately enters general circulation. Vitamin C generally circulates as ascorbic acid.
Query \(\PageIndex{3}\)
Accumulation
Most water-soluble vitamins are not stored in the body. Vitamin C is not stored, but is accumulated in certain tissues in the body where it can be 5-100 times higher than found in the plasma1. The table below shows the concentrations of vitamin C in different tissues and fluids.
| Organ/Tissue | Vitamin C Concentration* | Organ/Tissue | Vitamin C Concentration* |
|---|---|---|---|
| Pituitary Gland | 40-50 | Lungs | 7 |
| Adrenal Gland | 30-40 | Skeletal Muscle | 3-4 |
| Eye Lens | 25-31 | Testes | 3 |
| Liver | 10-16 | Thyroid | 2 |
| Brain | 13-15 | Cerebrospinal Fluid | 3.8 |
| Pancreas | 10-15 | Plasma | 0.4-1 |
| Spleen | 10-15 | Saliva | 0.1-9.1 |
| Kidneys | 5-15 |
* mg/100 g wet tissue, mg/100 mL fluids
How does the body accumulate such high levels of vitamin C? There are 2 primary mechanisms:
- Ascorbic Acid (Ascorbate) uptake using sodium-dependent vitamin C transporter (SVCT) 1 or 2
- Ascorbic Acid (Ascorbate) Recycling
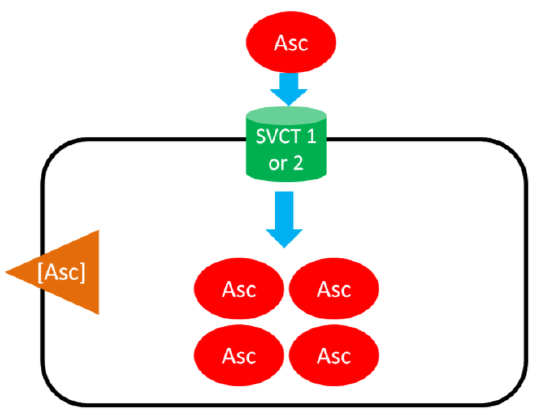
Ascorbic Acid (Ascorbate) transport using sodium-dependent vitamin C transporter (SVCT) 1 or 2
As shown below, SVCT 1 and SVCT 2 transport ascorbic acid or ascorbate into the cell against the concentration gradient (represented by the orange wedge in the figure below). Like absorption, this uptake is driven by the action of sodium-potassium ATPase. This mechanism is saturable, meaning that at high concentrations it reaches a threshold where it cannot take up ascorbic acid any faster. Thus, there is a limit to how much can be taken up through this mechanism7.
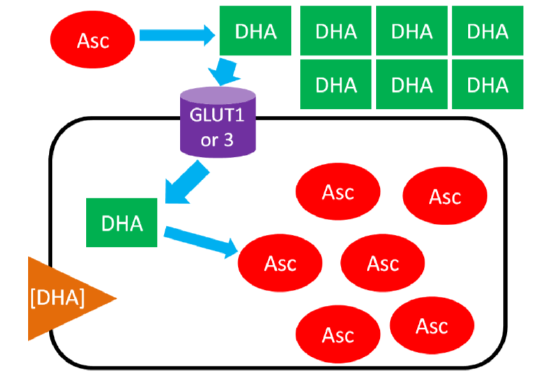
Ascorbic Acid (Ascorbate) Recycling
In ascorbic acid recycling, ascorbic acid is oxidized to dehydroascorbic acid (DHA). DHA is then transported into the cell moving with its concentration gradient using GLUT1 or 3. Once inside the cell, DHA is reduced back to ascorbic acid, thus maintaining the DHA gradient. As a result, the cell is able to accumulate high levels of ascorbic acid6. The figure below depicts ascorbic acid recycling.
Query \(\PageIndex{4}\)
Enzymatic Functions
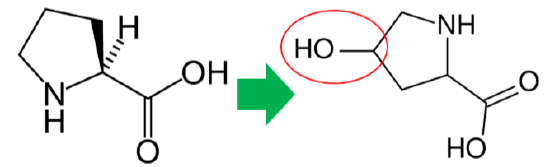
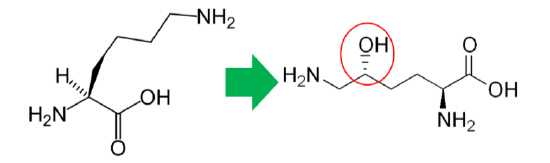
In addition to its antioxidant function, vitamin C is also a cofactor for a number of enzymes or contributes to reducing a cofactor in others. Proline hydroxylase and lysyl hydroxylase are two enzymes that vitamin C is needed for them to carry out their catalytic function. These enzymes are important in the formation of the protein collagen. Hydroxylase means that the enzymes add alcohol (hydroxyl, \(\ce{-OH}\)) to the amino acids proline and lysine, as shown below.
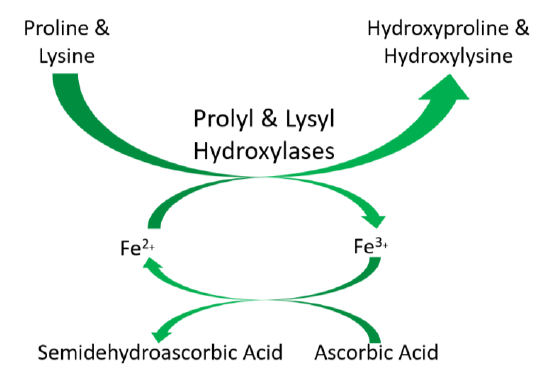
As shown below, prolyl and lysyl hydroxylases require ferrous iron (\(\ce{Fe^2+}\)) to function. But in the course of the hydroxylating proline or lysine, ferrous iron (\(\ce{Fe^2+}\)) is oxidized to ferric iron (\(\ce{Fe^3+}\)). Ascorbic acid is required to reduce \(\ce{Fe^3+}\) to \(\ce{Fe^2+}\), forming semidehydroascorbic acid in the process. With \(\ce{Fe^2+}\), the enzyme is then able to continue to hydroxylate proline and lysine.
Query \(\PageIndex{5}\)
Query \(\PageIndex{6}\)
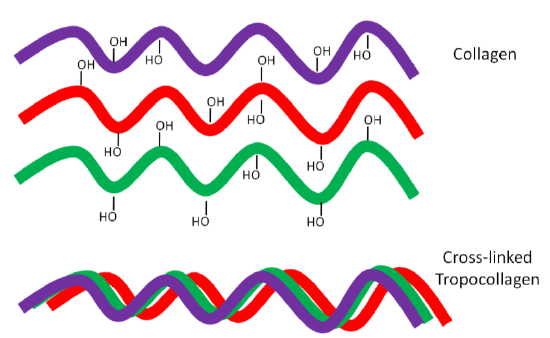
Why should you care about collagen formation? Because collagen is estimated to account for 30% or more of total body proteins11. Collagen contains a number of hydroxylated prolines and lysines that are needed for collagen strands to properly cross-link. This cross-linking is important for collagen to wind together like a rope, forming the strong triple helix known as tropocollagen. This process is shown in the following animation, as well as in the figure below.
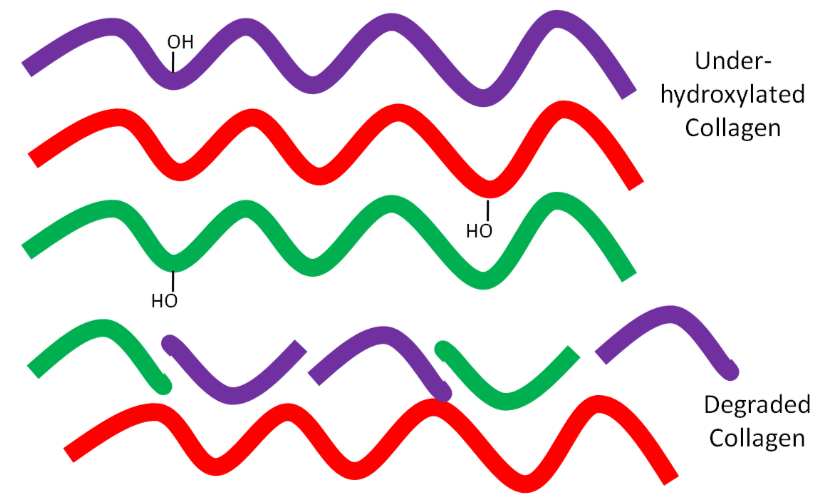
But if there is not enough ascorbic acid available, the collagen strands are underhydroxylated and instead of forming strong tropocollagen, the underhydroxylated collagen is degraded as shown below.
This weak collagen then results in the symptoms seen in the vitamin C deficiency, scurvy, that will be discussed in the next subsection.
Ascorbic Acid is also needed for:
- Carnitine synthesis
- Tyrosine synthesis and catabolism
- Serotonin (neurotransmitter) synthesis
- Other hormone and neurotransmitter synthesis12
The figure below shows how ascorbic acid is needed for dopamine hydroxylase, which ultimately produces the hormone epinephrine.

Query \(\PageIndex{7}\)
Vitamin C Deficiency (Scurvy)
Why should you care about the details of prolyl and lysyl hydroxylases and their actions on collagen hydroxylation and tropocollagen formation? Because they explain the symptoms of vitamin C deficiency. While it is rare in the United States, vitamin C deficiency, known as scurvy, displays symptoms that are a result of weak tropocollagen, that in turn, weakens connective tissue throughout the body. Symptoms of scurvy include bleeding gums, pinpoint hemorrhages, and corkscrew hairs as shown in the figure and link below.
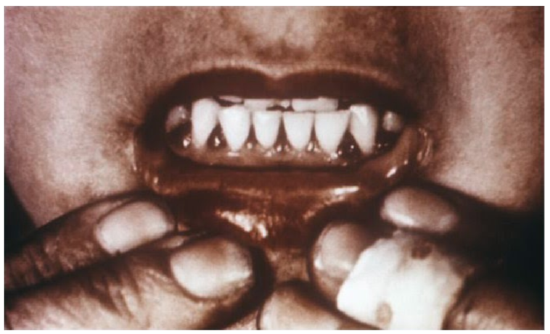
Web Link
Query \(\PageIndex{8}\)
Additional symptoms include impaired wound and fracture healing, easy bruising, and loose or decaying teeth. Scurvy can be fatal if not treated. Scurvy was the first discovered nutrition deficiency in 1746 by James Lind, who is shown below12.

Keep in mind as you read the description below that suggesting scurvy was due to a diet deficiency, at that time, sounded like flu was caused by not consuming green beans. Sounds pretty crazy, right? Because the concept of vitamines and compounds that would become vitamins were not proposed until around 150 years later.
Lind was a surgeon on a British navy ship. Frequently during voyages the sailors would develop scurvy for reasons that were not understood at the time. It was known that citrus fruits could cure or prevent scurvy, but it was believed this was due to their acidity. Lind performed clinical trials comparing citrus juice to dilute sulfuric acid and vinegar and found that only citrus juice caused the sailors to recover, as depicted in the link below. As a result of the discovery, the British sailors became known as "Limeys" because they would drink lime juice to prevent the development of the disease16.
Web Link
Query \(\PageIndex{9}\)
Vitamin C Toxicity, Linus Pauling & the Common Cold
Vitamin C does not have a toxicity per se, but in some people over 2 grams/day can lead to diarrhea and gastrointestinal distress. In addition, high intake of vitamin C increases excretion of uric acid (urate) and oxalic acid (oxalate). The structure of these 2 compounds are shown below.
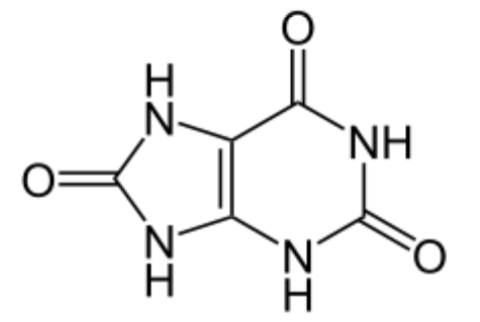
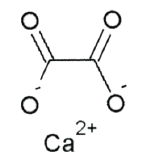
These compounds are the primary components of 2 types of kidney stones21. The figures below show the most common sites of pain in someone with kidney stones.
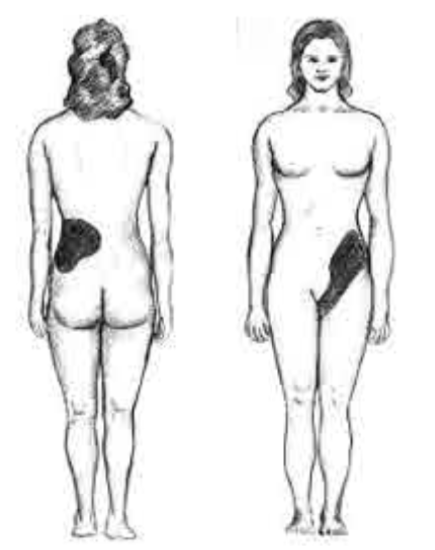
The following video describes what kidney stones are and the symptoms that can occur if someone has kidney stones. The link shows some pictures of kidney stones.
Web Link
Calcium oxalate is one of the primary forms of kidney stones with uric acid stones being more rare20. However, a link between excretion of these compounds and actual stone formation has not been established. Nevertheless, high-dose vitamin C supplementation should be approached with some caution, since it is not clear whether it increases the risk of forming kidney stones21.
Query \(\PageIndex{10}\)
Linus Pauling and the Common Cold
The person who popularized taking megadoses of vitamin C was Dr. Linus Pauling. Dr. Pauling was a chemist, and is the only person to receive 2 unshared Nobel Prizes. The Nobel Prize is a prestigious award, and Dr. Pauling was close to solving the structure of DNA. This would have likely netted him another Nobel prize, but Watson and Crick beat him to it.

Later in his life Pauling became convinced that megadoses of vitamin C could prevent the common cold. In 1970 his book Vitamin C and the Common Cold was released and became a bestseller. Later he came to believe that vitamin C could prevent cardiovascular disease, cancer, and combat aging23. However, critics of his beliefs countered that all megadose supplementation was doing was creating "expensive urine". This refers to the fact that the RDA is only 75-90 mg/day for adults and Pauling recommended taking 1-2 grams of vitamin C daily24. Thus, with vitamin C being water-soluble, most of the vitamin C that people on the regimen were paying to consume was being excreted in the urine, thus making it “expensive”.
A review of vitamin C and colds found that that routine megadoses of vitamin C do not reduce the risk of the common cold in most individuals. However, there is some evidence that it might benefit people exposed to brief periods of severe physical exercise (marathon runners) or cold environments (skiers and soldiers in subarctic conditions). There has been little research conducted in children, so it is not known whether vitamin C supplementation is beneficial in this age group25.
References
- Stipanuk MH. (2006) Biochemical, physiological, & molecular aspects of human nutrition. St. Louis, MO: Saunders Elsevier.
- en.Wikipedia.org/wiki/File:As..._structure.png
- en.Wikipedia.org/wiki/File:De...orbic_acid.png
- Packer L, Weber SU, Rimbach G. (2001) Molecular aspects of alpha-tocotrienol antioxidant action and cell signalling. J Nutr 131(2): 369S-373S.
- Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, editors. (2006) Modern nutrition in health and disease. Baltimore, MD: Lippincott Williams & Wilkins.
- Li Y, Schellhorn H. (2007) New developments and novel therapeutic perspectives for vitamin C. J Nutr 137(10): 2171-2184.
- en.Wikipedia.org/wiki/Prolin..._-_Proline.svg
- en.Wikipedia.org/wiki/File:Hy..._structure.svg
- en.Wikipedia.org/wiki/Lysine...D-skeletal.png
- en.Wikipedia.org/wiki/File:Hydroxylysine.png
- Di Lullo G, Sweeney S, Korkko J, Ala-Kokko L, San Antonio J. (2002) Mapping the ligand-binding sites and disease-associated mutations on the most abundant protein in the human, type I collagen. The Journal of Biological Chemistry 277(6): 4223-4231.
- Gropper SS, Smith JL, Groff JL. (2008) Advanced nutrition and human metabolism. Belmont, CA: Wadsworth Publishing.
- en.Wikipedia.org/wiki/File:Ca...osynthesis.svg
- en.Wikipedia.org/wiki/File:Scorbutic_gums.jpg
- en.Wikipedia.org/wiki/File:James_lind.jpg
- Carpenter K. (2003) A short history of nutritional science: Part 3 (1912-1944). J Nutr 133(10): 638-645.
- en.Wikipedia.org/wiki/File:Ha...e_Ketoform.svg
- en.Wikipedia.org/wiki/File:Calcium_oxalate.png
- https://www.niddk.nih.gov/health-inf.../kidney-stones
- commons.wikimedia.org/wiki/File:Pos-renal.png
- Massey L, Liebman M, Kynast-Gales S. (2005) Ascorbate increases human oxaluria and kidney stone risk. J Nutr 135(7): 1673-1677.
- https://www.flickr.com/photos/oregon...ity/5711642694
- http://lpi.oregonstate.edu/lpbio/lpbio2.html
- http://www.health.harvard.edu/newsle..._linus_pauling
- Hemilä H, Chalker E. (2013) Vitamin C for preventing and treating the common cold. Cochrane Database of Systematic Reviews. 31(1):CD000980. doi: 10.1002/14651858.CD000980.pub4.


