Aspartame
- Page ID
- 5916
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)In 1965, Jim Schlatter, a chemist at G.D. Searle was working on a on a project to discover new treatments for gastric ulcers. One of the steps in the research process was to make a dipeptide intermediate, aspartyl-phenylalanine methyl ester. He accidently and unknownly spilled some on his hand. Later he licked his finger as he reached for a piece of paper (unsanitary lab technique), and noticed the sweet taste. He and a friend decided to test some in coffee and confirmed the identify of the chemical with the sweet taste. The result was the sweetner, aspartame, sold under the tradmark names of NutraSweet and Equal.
Introduction
Aspartame has a sweet taste with minimal bitterness. Its onset of sweetness may be slightly slower than sucrose, and the sweetness may linger. The sweetness potency relative to sucrose is about 180. Aspartame is metabolized in the body to its components: aspartic acid, phenylalanine, and methanol. Like other amino acids, it provides 4 calories per gram. Since it is about 180 times as sweet as sugar, the amount of aspartame needed to achieve a given level of sweetness is less than 1% of the amount of sugar required. Thus 99.4% of the calories can be replaced.
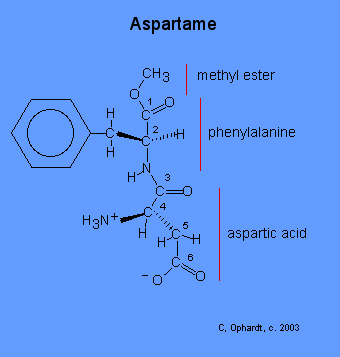
Synthesis
Two of the components of aspartame (phenylalanine and aspartic acid) are chiral, which means that they have two isomers that are non-superimposable mirror images. If the incorrect isomers are used, the aspartame molecule will not have the correct shape to fit the binding site of the 'sweetness' receptors on the tongue.
In the synthesis of aspartame, the starting materials are a racemic mixture (equal quantities of both isomers) of phenylalanine, and aspartic acid. Only the L isomer of phenylalanine is desired for use. This L isomer may be separated from the D isomer by a chemical pretreatment, followed by a reaction with the enzyme porcine kidney acylase. The final separation occurs with an acidic aqueous and organic extraction, where the L isomer is more soluble in the aqueous layer and the D isomer is more soluble in the organic layer.
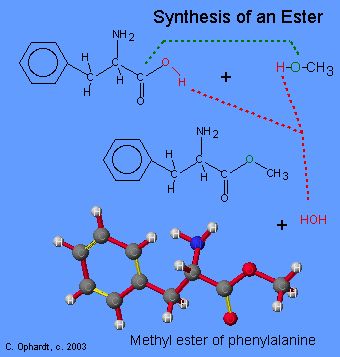
Ester Synthesis
Treatment of L-phenylalanine with methanol in the presence hydrochloric acid causes an ester reaction with the acid group on the phenylalanine. The product is the methyl ester of phenylalanine.
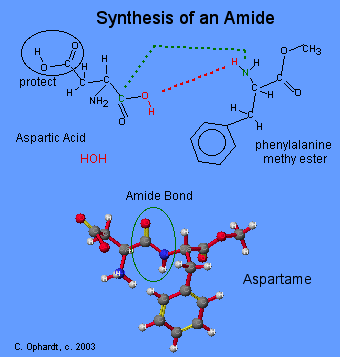
Amide Synthesis
The final goal is to react the methyl ester of phenylalamine with aspartic acid to give the dipeptide amide structure. A series of reactions is required so that only the acid of the base amino acid is reacted. The acid group on the side chain is protected so that it does not react.
Safety Concerns
Many years were spent testing the product as required by the FDA. In the almost two decades since first approval, testing continues and the use of NutraSweet remains a rather controversial issue. A search of the Internet will reveal many anecdotal but scientifically undocumented bulletins regarding health risks associated with the use of NutraSweet. Phenylalanine is one of the "essential" amino acids, meaning that humans must get it from their diet. It is a precursor for the synthesis of tyrosine and several neurotransmitters. Excess phenylalanine is broken down to fumarate and acetoacetate, both of which are part of normal energy metabolism.
People who lack the enzyme to convert phenylalanine to tyrosine are not able to metabolize phenylalanine normally. This condition is called phenylketonuria, PKU, because excess phenylalanine is instead converted to phenylketones which appear in the urine. If it is not detected and treated, this condition can lead to mental retardation. This was the first genetic disease for which a routine screening test became available. Persons having this genetic defect must monitor their intake of phenylalanine. For this reason, products containing aspartame carry an information label for phenylketonurics.

