9.5: Electrolytes Important for Fluid Balance
- Page ID
- 80570
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Moving Electrolytes Across Membranes
Cells constantly transport nutrients in and waste out, but this constant movement of molecules could cause too much water to move in or out of the cells. So, how is the concentration of solutes maintained to achieve water balance? To maintain homeostasis, the cell creates proteins that transport molecules, particularly the electrolytes sodium, chlorine, and potassium.
For example, the sodium-potassium pump, a protein found in a variety of human cells, uses energy to pump sodium ions out of the cell while moving potassium ions into the cell. The constant work of the sodium-potassium pump maintains the solute equilibrium and, consequently, water distribution between intracellular and extracellular fluids. The sodium-potassium pump is also critical for the function of nerve and muscle cells. The unequal movement of the positively charged sodium (Na+) and potassium (K+) ions makes intracellular fluid more negatively charged than the extracellular fluid. This charge gradient is another source of energy that a cell uses to perform work and is essential for nerve conduction and muscle contraction. However, the sodium-potassium pump uses energy to pump electrolytes, and nearly half of the brain's energy is spent on maintaining the sodium-potassium pump. In this section, we will further explore the sources and functions of sodium, chlorine, and potassium.
Video \(\PageIndex{1}\): The sodium-potassium pump is the primary mechanism for cells to maintain water balance between themselves and their surrounding environment.1 Source: https://www.youtube.com/watch?v=AkiaMiGnPuQ
Sodium
Functions of Sodium in the Body
You have probably heard that most Americans eat too much sodium. As a component of table salt, sodium can be abundant in the diet because it is an effective preservative and flavor enhancer. Although excess sodium is linked to health problems, sodium is an essential nutrient that functions in water balance, nerve signaling, and muscle contraction. Sodium is also essential for nutrient absorption in the small intestine and nutrient reabsorption in the kidney. Amino acids, glucose, and water must go from the small intestine to the blood. To do so, they pass through intestinal cells on their way to the blood. The transport of nutrients through intestinal cells is facilitated by the sodium-potassium pump, which moves sodium out of the cell and creates a higher sodium concentration outside the cell.
Sodium Requirements and Recommendations
In contrast to many minerals, sodium absorption in the small intestine is extremely efficient. In a healthy individual, 90-95% of sodium is excreted by the kidneys, and the remainder is lost in feces and sweat. The kidneys actively reabsorb sodium, so ultimately, dietary sodium is only needed in small amounts. The Food and Nutrition Board of the National Academies of Science, Engineering, and Medicine set an Adequate Intake (AI) level for sodium for healthy adults between the ages of nineteen and fifty at 1.5 grams (1,500 milligrams) per day. The AI takes into account the amount of sodium lost in sweat during recommended physical activity levels and additionally provides for the sufficient intake of other nutrients, such as chloride. There is no tolerable upper intake level established for sodium because there is not enough evidence demonstrating toxic effects of sodium, other than chronic diseases such as hyptertension.2
The Food and Nutrition Board more recently established a Chronic Disease Risk Reduction (CDRR) Intake level for sodium based on many studies focused on determining the association between reduced sodium intake and cardiovascular disease and high blood pressure, also known as hypertension. Based on the evidence, the CDRR intake for sodium is set to 2.3 grams (2300 milligrams) per day for adults. Reducing sodium intake below the CDDR will likely lower the risk of chronic diseases in the general population.2 Table salt is approximately 40 percent sodium and 60 percent chloride, and one teaspoon of salt contains approximately 2.3 grams (2300 milligrams) of sodium (Figure \(\PageIndex{1}\)). The average American consumes 3.4 grams (3400 milligrams) of sodium each day, which is much more than the recommended amounts.3

Figure \(\PageIndex{1}\): Table salt. One teaspoon of table salt provides the Chronic Disease Risk Reduction daily limit for sodium. Exceeding this amount of salt may lead to health consequences like hypertension and cardiovascular disease. Source: "Salt and spoon" by Bill HR is licensed under CC BY 2.0.
Food Sources of Sodium
Most sodium in the typical American diet comes from processed and prepared foods (Figure \(\PageIndex{2}\)). Manufacturers add salt to foods to improve texture and flavor and also as a preservative. The amount of salt in similar food products varies widely. Some foods, such as meat, poultry, and dairy, contain naturally-occurring sodium. For example, one cup of low-fat milk contains 107 milligrams of sodium. Naturally-occurring sodium accounts for less than 12 percent of dietary intake in a typical diet. Soft water replaces calcium with sodium, which contributes to the sodium content of one's diet. Soy sauce and monosodium glutamate are also sources of sodium.3
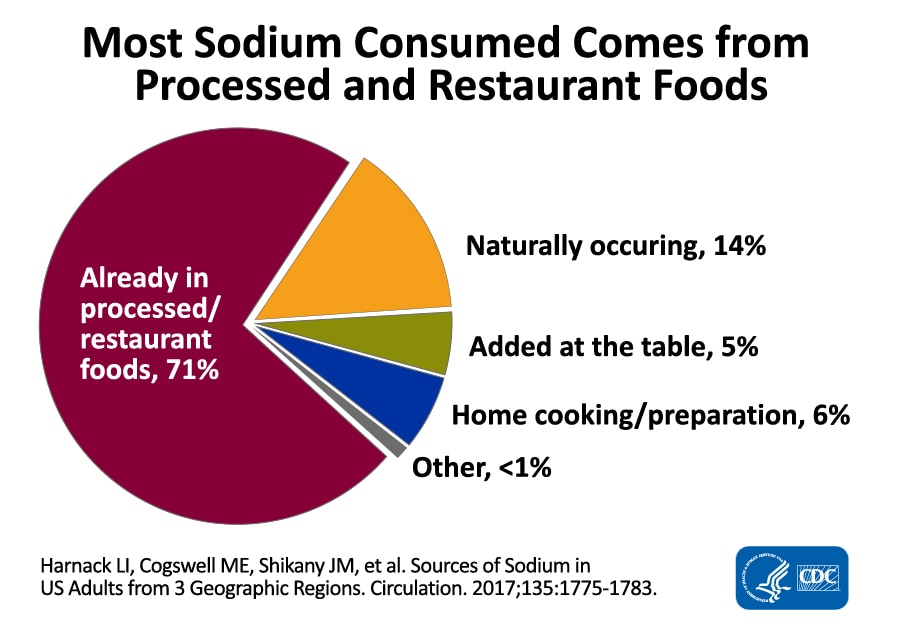
Figure \(\PageIndex{2}\): Dietary Sources of Sodium. Most sodium is consumed in processed foods and prepared foods. "Sodium Chart" by the Centers for Disease Control and Prevention is in the Public Domain.
Sodium Excess and Deficiency
In the United States, sodium deficiency is rare because table salt is commonly and abundantly added to foods. In certain circumstances, however, low blood sodium levels can occur. This condition, known as hyponatremia, can occur when taking certain medications that affect kidney function or when water balance is disrupted due to vomiting, diarrhea, or even excessive water consumption. Symptoms of hyponatremia include nausea, headache, confusion, and seizures, depending on the severity of the condition.4
Health complications due to excessive sodium intake are much more common. If there is excessive sodium in the diet, the kidneys cannot efficiently remove sodium from the blood. Extremely high levels of sodium in the blood lead to hypernatremia. Symptoms include excessive thirst, fatigue, and confusion. When sodium levels increase in the blood, water moves out of cells into the blood plasma and interstitial fluid. This increases the pressure in the blood, a condition called hypertension, and requires more energy from the heart to continue pumping blood through the body. This extra work and pressure on the blood vessels can lead to heart attacks or strokes.
Many studies have shown that reducing sodium intake can help prevent hypertension and possible cardiovascular disease. For example, a large 8-year study called the Trials of Hypertension Prevention found that reduced sodium intake was associated with reductions in blood pressure throughout the study. After 10-15 years, individuals who reduced sodium intake were 25% less likely to have a heart attack, stroke, bypass surgery, or have died from heart disease.5,6 Although the direct connection between sodium intake and cardiovascular disease needs more research, hypertension is a leading cause of cardiovascular disease.7
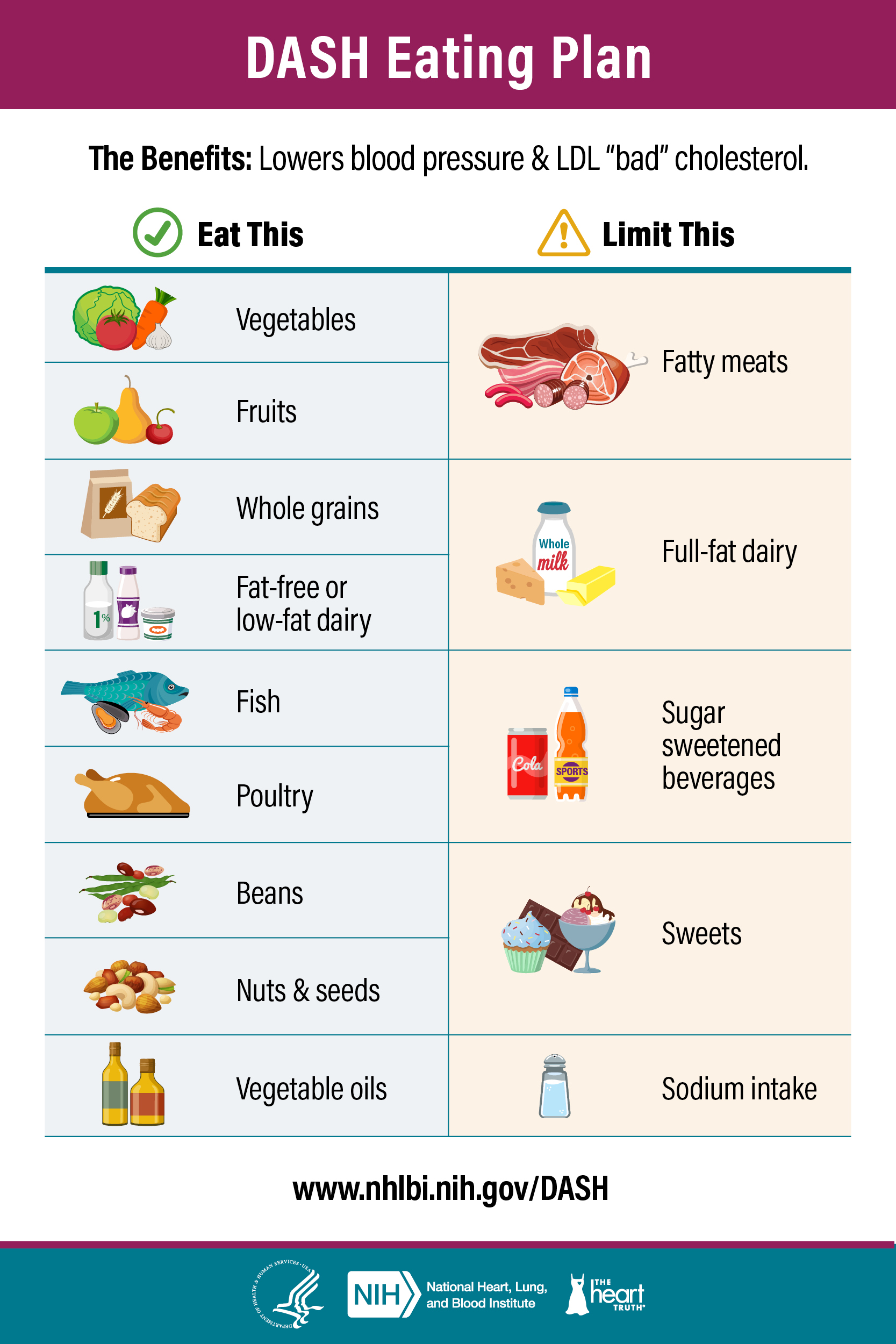
The Dietary Approaches to Stop Hypertension (DASH) diet was first described in 1997 as a way to reduce hypertension. In the first study of the diet, 456 people were randomly assigned to one of three diets: 1) the average American diet with red meat, sugars, and low fiber; 2) a similar diet with fruits and vegetables; and 3) a DASH diet consisting of mostly fruits, vegetables, and low-fat dairy with limited sugars, red meat, and saturated fats.8 After 8 weeks, the individuals on the DASH diet had the greatest reduction in blood pressure. Later studies showed that a low-sodium DASH diet had an even greater effect.9 Even more recently, a review of multiple studies reaffirmed the value of the DASH diet on lowering blood pressure, as well as with weight loss and reducing the risk of Type 2 diabetes.10 The image below summarizes the DASH diet components, and you can find information to help follow the DASH diet at the National Heart, Lung, and Blood Institute's DASH Eating Plan.
Figure \(\PageIndex{2}\): DASH Eating Plan from the National Heart, Lungs, and Blood Institute. Source11: https://nhlbi.nih.gov/education/dash-eating-plan "Dash Eating Plan" by the National Heart, Lungs, and Blood Institute is in the Public Domain.
Salt Sensitivity
High dietary intake of sodium is one risk factor for hypertension and contributes to high blood pressure in many people. However, studies have shown that not everyone’s blood pressure is affected by lowering sodium intake. About a third of the population is salt-sensitive, meaning their blood pressure is affected by salt intake.12 Genetics, race, sex, weight, and physical activity level are determinants of salt sensitivity. African Americans, women, and overweight individuals are more salt-sensitive than others. Also, if hypertension runs in a person’s family, that person is more likely to be salt-sensitive.
Chloride
Functions of Chloride in the Body
Chloride is the primary negatively charged electrolyte or anion in extracellular fluid. Chloride plays a role in fluid balance as it moves across membranes following sodium and potassium gradients. Chloride also has its own protein channels that reside in cell membranes. These protein channels are especially abundant in the gastrointestinal tract, pancreas, and lungs. Chloride is the major anion in sweat and vomit. It is the anion of stomach acid, which is hydrochloric acid.
Chloride channels play a role in regulating fluid secretion, such as pancreatic juice into the small intestine and the flow of water into mucus. The importance of chloride movement in the body is exemplified in the signs and symptoms of a genetic disease called cystic fibrosis. This condition is caused by a mutation in a protein that transports chloride ions out of the cell. Signs and symptoms of this condition include salty skin, poor digestion and absorption, sticky mucus accumulation in the lungs, and liver damage. The digestive process is impaired because chloride is not transported properly, resulting in sticky mucus that blocks ducts in the pancreas, thereby preventing pancreatic juices from reaching the digestive tract.
Chloride Requirements and Recommendations
Table salt is 60% chloride, so most chloride in the diet comes from salt. A teaspoon of salt equals 5.6 grams, with each teaspoon of salt containing 3.4 grams of chloride and 2.2 grams of sodium. The Adequate Intake (AI) level of chloride AI for adults is 2.3 grams.13 Therefore, just ⅔ teaspoon of table salt per day is sufficient for chloride, as well as sodium.
Food Sources of Chloride
Chloride has dietary sources other than table salt, namely as another form of salt—potassium chloride. Dietary sources of chloride are all foods containing sodium chloride, as well as tomatoes, lettuce, olives, celery, rye, whole-grain foods, and seafood. Although many salt substitutes are sodium-free, they may still contain chloride.
Chloride Excess and Deficiencies
Low dietary intake of chloride and more often diarrhea can cause low blood levels of chloride. Symptoms typically are similar to those of hyponatremia and include weakness, nausea, and headache. Excess chloride in the blood is rare with no characteristic signs or symptoms.
Potassium
Functions of Potassium in the Body
Potassium is the most abundant positively charged electrolyte inside cells. Ninety percent of potassium exists in intracellular fluid, with about 10 percent in extracellular fluid, and only 1 percent in blood plasma. As with sodium, potassium levels in the blood are strictly regulated. When potassium levels in the blood increase, the adrenal glands release a hormone that stimulates an increase in the number of sodium-potassium pumps in the kidneys. Sodium is then reabsorbed, and more potassium is excreted. Because potassium is required for maintaining sodium levels, and hence fluid balance, about 200 milligrams of potassium are lost from the body every day. So, one of potassium's important functions is fluid and electrolyte balance. Potassium is also required for nerve signaling, protein synthesis, and energy metabolism and acts as a buffer in blood, playing a role in acid-base balance.
Potassium Requirements and Recommendations
According to the U.S. Dietary Reference Intakes, there is not enough evidence to establish a Recommended Dietary Allowance (RDA) for potassium. However, the National Academy of Medicine has established an Adequate Intake (AI) for potassium based on the levels associated with a decrease in blood pressure, a reduction in salt sensitivity, and a minimal risk of kidney stones. For adult male and females above the age of nineteen, the adequate intake for potassium is 3.4 grams (3400 milligrams) and 2.6 grams (2600 milligrams) per day, respectively. Unlike sodium, the Food and Nutrition Board did not find sufficient evidence to recommend a CDRR value for potassium.2 Most Americans do not consume enough potassium in the diet, and potassium is considered a nutrient of public health concern according to the Dietary Guidelines for Americans.12 Based on survey data, the average daily potassium intake from foods is 3,016 mg for men and 2,320 mg for women aged 20 and older. Because of this, potassium is now listed of food nutrition labels to help consumers improve potassium intake. Health concerns associated with insufficient potassium intakes include increased blood pressure, risk for kidney stones, bone turnover, urinary calcium excretion, and salt sensitivity.
Many foods contain potassium, but it is important to have a varied diet to achieve the recommended potassium intake. Take some time to find one vegetable, fruit, dairy, and protein-rich food that contains potassium using the Dietary Guidelines for Americans Potassium Food Sources page.14
Imbalances of Potassium
Insufficient potassium levels in the body, known as hypokalemia, can be caused by a low dietary intake of potassium or by high sodium intakes, but more commonly, it results from medications that increase water excretion, mainly diuretics. The signs and symptoms of hypokalemia are related to the functions of potassium in nerve cells and, consequently, skeletal and smooth muscle contraction. The signs and symptoms include muscle weakness and cramps, respiratory distress, and constipation. Severe potassium depletion can cause the heart to have abnormal contractions and can even be fatal. High levels of potassium in the blood, or hyperkalemia, is toxic and also affects the heart. It is a silent condition as it often displays no signs or symptoms. Extremely high potassium levels in the blood disrupt the electrical impulses that stimulate the heart and can cause the heart to stop. Hyperkalemia is usually the result of kidney dysfunction.
Groups at risk of developing hypokalemia are individuals with renal and large intestine issues such as urine and diarrhea losses; people with uncontrolled diabetes, which causes diabetic acidosis and urine losses; dehydration; prolonged diarrhea or emesis; and certain drugs like diuretics, steroids, or laxatives. These deficiencies are due to excessive loss and not a dietary deficiency.
Attributions
- Zimmerman, "An Introduction to Nutrition (Zimmerman)," CC BY-NC-SA 3.0. Text was updated and reorganized. Figures and emphasis boxes were added.
References
- Neuroscientifically Challenged. 2-Minute Neuroscience: Sodium-Potassium Pump. [Video]. YouTube. https://youtu.be/AkiaMiGnPuQ?si=yVyJT126cIccVBNX. Published November 20, 2019. Accessed October 12, 2023.
- Stallings VA, Quirk M, Oria M, eds. Dietary Reference Intakes for Sodium and Potassium: A Consensus Study Report of The National Academies of Sciences, Engineering, Medicine. Washington, DC: The National Academies Press; 2019. https://nap.nationalacademies.org/re...5353/chapter/1. Accessed October 2, 2023.
- About Sodium and Health. Centers for Disease Control and Prevention. Updated January 31, 2024. Accessed October 3, 2023. https://www.cdc.gov/salt/index.htm.
- Hyponatremia. Mayo Clinic. Published May 17, 2022. Accessed October 5, 2023. https://www.mayoclinic.org/diseases-...s/syc-20373711.
- Cook NR, Cutler JA, Obarzanek E, Buring JE, et. al. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP). BMJ. 2007;334:885. doi:10.1136/bmj.39147.604896.55.
- Cook NR, Obarzanek E, Cutler JA, et al. Joint effects of sodium and potassium intake on subsequent cardiovascular disease: the trials of hypertension prevention follow-up study. Arch Intern Med. 2009;169(1):32-40. doi:10.1001/archinternmed.2008.523.
- He FJ, MacGregor GA. A comprehensive review on salt and health and current experience of worldwide salt reduction programmes. J Hum Hypertens. 2009;23(6):363-384 doi.org/10.1038/jhh.2008.144.
- Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med. 1997;336(16):1117-1124. doi:10.1056/NEJM199704173361601.
- Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med. 2001;344(1):3-10. doi:10.1056/NEJM200101043440101.
- The Science Behind the DASH Eating Plan. NIH National Heart, Lung, and Blood Institute. nhlbi.nih.gov. Updated December 29, 2021. Accessed October 16, 2023. https://www.nhlbi.nih.gov/education/dash/research.
- DASH Eating Plan. NIH National Heart, Lung, and Blood Institute. Updated December 29, 2021. Accessed October 16, 2023. https://www.nhlbi.nih.gov/education/dash-eating-plan.
- Bailey MA, Dhaun N. Salt sensitivity: causes, consequences, and recent advances. Hypertension. 2023;81:1-14. doi:10.1161/HYPERTENSIONAHA.123.17959.
- Potassium: Fact Sheet for Health Professionals. NIH office of dietary supplements. Updated June 2, 2022. Accessed October 17, 2023. https://ods.od.nih.gov/factsheets/Po...ofessional/#h5.
- Food Sources of Potassium. Dietary Guidelines for Americans. Accessed October 1, 2023. https://www.dietaryguidelines.gov/fo...rces-potassium.