1.3: The Classification of Matter
- Page ID
- 15189
- Use physical and chemical properties, including phase, to describe matter.
- Identify a sample of matter as an element, a compound, or a mixture.
Part of understanding matter is being able to describe it. One way chemists describe matter is to assign different kinds of properties to different categories.
Physical and Chemical Properties
The properties that chemists use to describe matter fall into two general categories. Physical properties are characteristics that describe matter. They include characteristics such as size, shape, color, and mass. These characteristics can be observed or measured without changing the identity of the matter in question. Chemical properties are characteristics that describe how matter changes its chemical structure or composition. An example of a chemical property is flammability—a material’s ability to burn—because burning (also known as combustion) changes the chemical composition of a material. The observation of chemical properties involves a chemical change of the matter in question, resulting in matter with a different identity and different physical and chemical properties.
Figure \(\PageIndex{1}\): (left) Ice Melting is a physical change. When liquid water (\(H_2O\)) freezes into a solid state (ice), it appears changed; However, this change is only physical as the the composition of the constituent molecules is the same: 11.19% hydrogen and 88.81% oxygen by mass. (right) Burning of wax to generate water and carbon dioxide is a chemical reaction. (CC-SA-BY-3.0; Andrikkos )Elements and Compounds
Any sample of matter that has the same physical and chemical properties throughout the sample is called a substance. There are two types of substances. A substance that cannot be broken down into chemically simpler components is called an element. Aluminum, which is used in soda cans and is represented by the symbol Al, is an element. A substance that can be broken down into chemically simpler components (because it consists of more than one element) is called a compound. Water is a compound composed of the elements hydrogen and oxygen and is described by the chemical formula, H2O. Today, there are about 118 elements in the known universe. In contrast, scientists have identified tens of millions of different compounds to date.
Sometimes the word pure is used to describe a substance, but this is not absolutely necessary. By definition, any single substance, element or compound is pure.
The smallest part of an element that maintains the identity of that element is called an atom. Atoms are extremely tiny; to make a line of iron atoms that is 1 inch long, you would need approximately 217 million iron atoms. The smallest part of a compound that maintains the identity of that compound is called a molecule. Molecules are composed of two or more different atoms that are attached together and behave as a unit. Scientists usually work with millions and millions of atoms and molecules at a time. When a scientist is working with large numbers of atoms or molecules at a time, the scientist is studying the macroscopic viewpoint of the universe. However, scientists can also describe chemical events on the level of individual atoms or molecules, which is referred to as the microscopic viewpoint. We will see examples of both macroscopic and microscopic viewpoints throughout this book (Figure \(\PageIndex{2}\)).
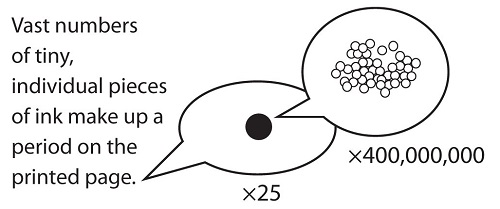 Figure \(\PageIndex{2}\): How Many Particles Are Needed for a Period in a Sentence? Although we do not notice it from a macroscopic perspective, matter is composed of microscopic particles so tiny that billions of them are needed to make a speck we can see with the naked eye. The ×25 and ×400,000,000 indicate the number of times the image is magnified.
Figure \(\PageIndex{2}\): How Many Particles Are Needed for a Period in a Sentence? Although we do not notice it from a macroscopic perspective, matter is composed of microscopic particles so tiny that billions of them are needed to make a speck we can see with the naked eye. The ×25 and ×400,000,000 indicate the number of times the image is magnified.
Mixtures
A material composed of two or more substances is a mixture. In a mixture, the individual substances maintain their chemical identities. Many mixtures are obvious combinations of two or more substances, such as a mixture of sand and water. Such mixtures are called heterogeneous mixtures. In some mixtures, the components are so intimately combined that they act like a single substance (even though they are not). Mixtures with a consistent or uniform composition throughout are called homogeneous mixtures (or solutions). For example, when sugar is dissolved in water to form a liquid solution, the individual properties of the components cannot be distinguished. Other examples or homogenous mixtures include solid solutions, like the metal alloy steel, and gaseous solutions, like air which is a mixture of mainly nitrogen and oxygen.
How would a chemist categorize each example of matter?
- saltwater
- soil
- water
- oxygen
- Answer a
-
Saltwater acts as if it were a single substance even though it contains two substances—salt and water. Saltwater is a homogeneous mixture, or a solution.
- Answer b
-
Soil is composed of small pieces of a variety of materials, so it is a heterogeneous mixture.
- Answer c
-
Water is a substance; more specifically, because water is composed of hydrogen and oxygen, it is a compound.
- Answer d
-
Oxygen, a substance, is an element.
How would a chemist categorize each example of matter?
- breakfast coffee
- hydrogen
- an egg
- Answer a
-
homogeneous mixture or solution
- Answer b
-
element
- Answer c
-
heterogeneous mixture
Phases or Physical States of Matter
All matter can be further classified by one of three physical states or phases, solid, liquid or gas. These three descriptions each imply that the matter has certain physical properties when in these states. A solid has a definite shape and a definite volume. Liquids ordinarily have a definite volume but not a definite shape; they take the shape of their containers. Gases have neither a definite shape nor a definite volume, and they expand to fill their containers.
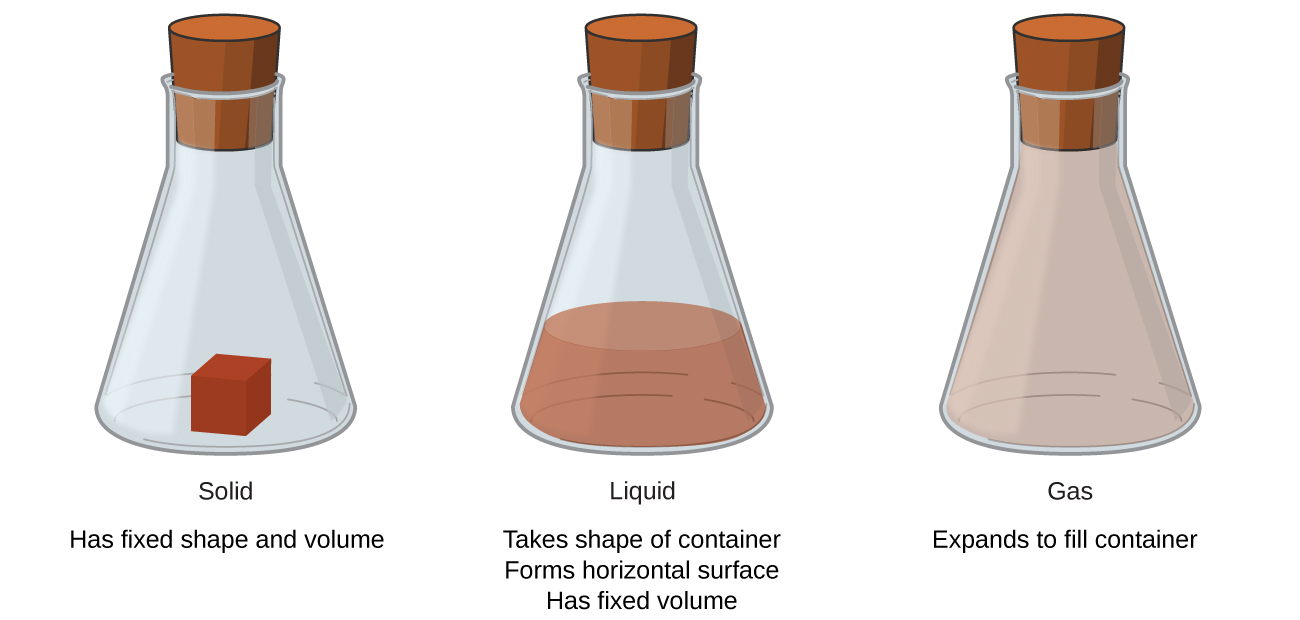 Figure \(\PageIndex{3}\): The three most common states or phases of matter are solid, liquid, and gas. (CC BY-4.0; OpenStax)
A beaker labeled solid contains a cube of red matter and says has fixed shape and volume. A beaker labeled liquid contains a brownish-red colored liquid. This beaker says takes shape of container, forms horizontal surfaces, has fixed volume. The beaker labeled gas is filled with a light brown gas. This beaker says expands to fill container.
Figure \(\PageIndex{3}\): The three most common states or phases of matter are solid, liquid, and gas. (CC BY-4.0; OpenStax)
A beaker labeled solid contains a cube of red matter and says has fixed shape and volume. A beaker labeled liquid contains a brownish-red colored liquid. This beaker says takes shape of container, forms horizontal surfaces, has fixed volume. The beaker labeled gas is filled with a light brown gas. This beaker says expands to fill container.
We encounter matter in each phase every day; in fact, we regularly encounter water in all three phases: ice (solid), water (liquid), and steam (gas) (Figure \(\PageIndex{2}\)).
 Figure \(\PageIndex{5}\): Boiling Water. When liquid water boils to make gaseous water, it undergoes a phase change. (CC BY-SA 3.0 Unported; Markus Schweiss via Wikipedia)
Figure \(\PageIndex{5}\): Boiling Water. When liquid water boils to make gaseous water, it undergoes a phase change. (CC BY-SA 3.0 Unported; Markus Schweiss via Wikipedia)
We know from our experience with water that substances can change from one phase to another if the conditions are right. Typically, varying the temperature of a substance (and, less commonly, the pressure exerted on it) can cause a phase change, a physical process in which a substance changes from one phase to another (Figure \(\PageIndex{5}\)). Phase changes are identified by particular names depending on what phases are involved, as summarized in Table \(\PageIndex{1}\).
| Change | Name |
|---|---|
| solid to liquid | melting, fusion |
| solid to gas | sublimation |
| liquid to gas | boiling, evaporation |
| liquid to solid | solidification, freezing |
| gas to liquid | condensation |
| gas to solid | deposition |
Figure \(\PageIndex{3}\) illustrates the relationships between the different ways matter can be classified.
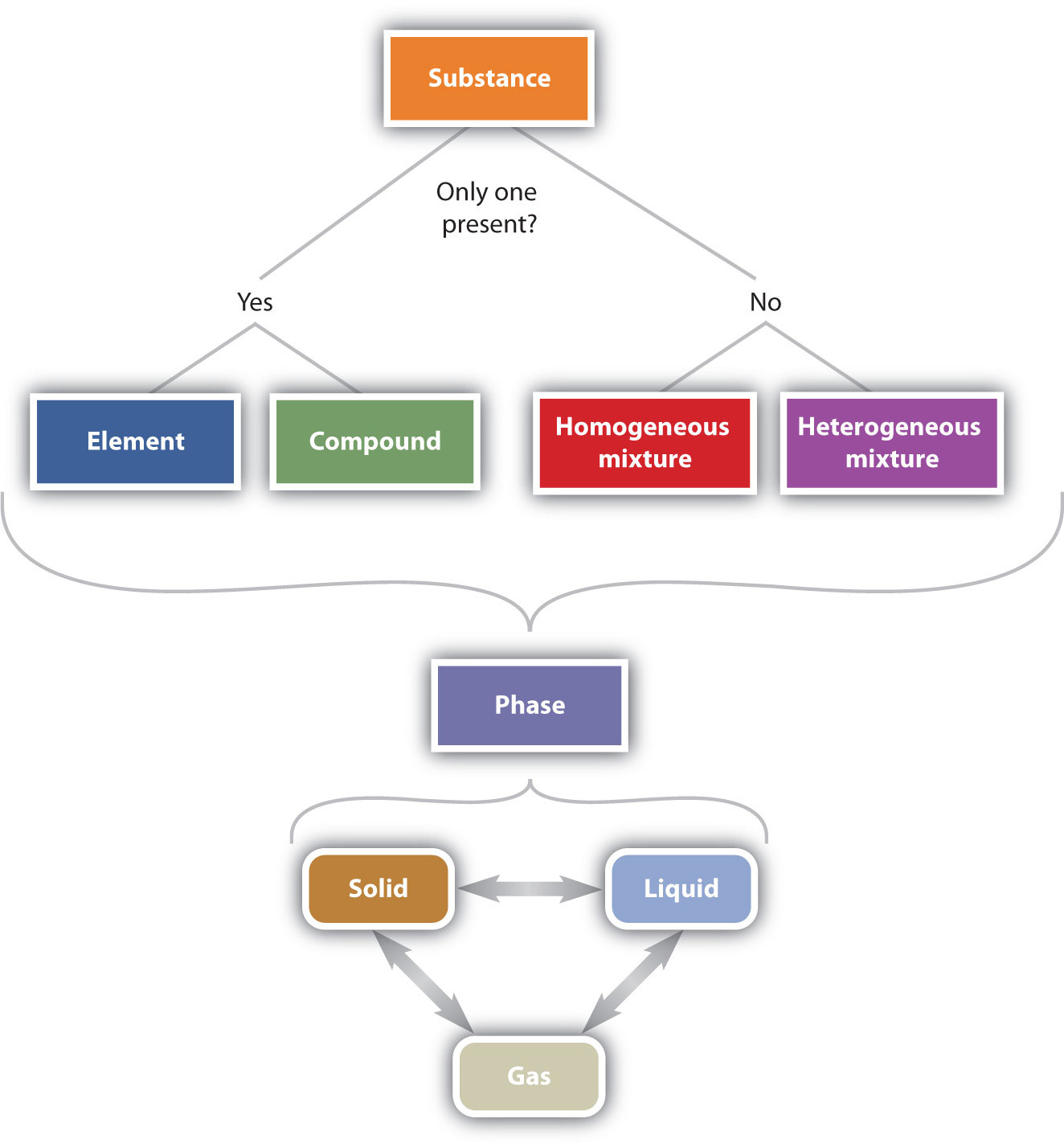 Figure \(\PageIndex{6}\): The Classification of Matter. Matter can be classified in a variety of ways, depending on its properties.
This table starts with a substance. If there is only one present, it can either be an element or a compound. If there is more than one present, it can either be a homogeneous mixture or a heterogeneous mixture. From these choices, the substance can be in a certain phase. It can either be solid, liquid, or gas, and each phase can go from one to another.
Figure \(\PageIndex{6}\): The Classification of Matter. Matter can be classified in a variety of ways, depending on its properties.
This table starts with a substance. If there is only one present, it can either be an element or a compound. If there is more than one present, it can either be a homogeneous mixture or a heterogeneous mixture. From these choices, the substance can be in a certain phase. It can either be solid, liquid, or gas, and each phase can go from one to another.
Concept Review Exercises
- Explain the differences between the physical properties of matter and the chemical properties of matter.
- What is the difference between a heterogeneous mixture and a homogeneous mixture? Give an example of each.
- Give at least two examples of a phase change and state the phases involved in each.
Answers
- Physical properties describe the existence of matter, and chemical properties describe how substances change into other substances.
- A heterogeneous mixture is obviously a mixture, such as dirt; a homogeneous mixture behaves like a single substance, such as saltwater.
- solid to liquid (melting) and liquid to gas (boiling) (answers will vary)
Key Takeaways
- Matter can be described with both physical properties and chemical properties.
- Matter can be identified as an element, a compound, or a mixture
Does each statement refer to a chemical property or a physical property?
- Balsa is a very light wood.
- If held in a flame, magnesium metal burns in air.
- Mercury has a density of 13.6 g/mL.
- Human blood is red.
- Answer
-
- physical property
- chemical property
- physical property
- physical property
Does each statement refer to a chemical property or a physical property?
- The elements sodium and chlorine can combine to make table salt.
- The metal tungsten does not melt until its temperature exceeds 3,000°C.
- The ingestion of ethyl alcohol can lead to disorientation and confusion.
- The boiling point of isopropyl alcohol, which is used to sterilize cuts and scrapes, is lower than the boiling point of water
- Answer
-
- chemical property
- physical property
- chemical property
- physical property
Define element. How does it differ from a compound?
- Answer
-
An element is a substance that cannot be broken down into chemically simpler components. Compounds can be broken down into simpler substances.
Define compound. How does it differ from an element?
- Answer
-
A compound is composed of two or more elements combined in a fixed ratio. An element is the simplest chemical substance.
Give two examples of a heterogeneous mixture.
- Answer
-
a salt and pepper mix and a bowl of cereal (answers will vary)
Give two examples of a homogeneous mixture.
- Answer
-
vinegar and rubbing alcohol (answers will vary)
Identify each substance as an element, a compound, a heterogeneous mixture, or a solution.
- xenon, a substance that cannot be broken down into chemically simpler components
- blood, a substance composed of several types of cells suspended in a salty solution called plasma
- water, a substance composed of hydrogen and oxygen
- Answer
-
- element
- heterogeneous mixture
- compound
Identify each substance as an element, a compound, a heterogeneous mixture, or a solution.
- sugar, a substance composed of carbon, hydrogen, and oxygen
- hydrogen, the simplest chemical substance
- dirt, a combination of rocks and decaying plant matter
- Answer
-
- compound
- element
- heterogeneous mixture
Identify each substance as an element, a compound, a heterogeneous mixture, or a solution.
- air, primarily a mixture of nitrogen and oxygen
- ringer’s lactate, a standard fluid used in medicine that contains salt, potassium, and lactate compounds all dissolved in sterile water
- tartaric acid, a substance composed of carbon, hydrogen, and oxygen
- Answer
-
- heterogeneous mixture
- solution
- compound
What word describes each phase change?
- solid to liquid
- liquid to gas
- solid to gas
- Answer
-
- melting or fusion
- boiling or evaporation
- sublimation
- What word describes each phase change?
- liquid to solid
- gas to liquid
- gas to solid
- Answer
-
- freezing
- condensation
- deposition



