2.3: Atomic Theory
- Page ID
- 15206
- Explain how all matter is composed of atoms.
- Describe the modern atomic theory.
- Recognize which elements exist as diatomic molecules.
Take some aluminum foil. Cut it in half. Now you have two smaller pieces of aluminum foil. Cut one of the pieces in half again. Cut one of those smaller pieces in half again. Continue cutting, making smaller and smaller pieces of aluminum foil.
It should be obvious that the pieces are still aluminum foil; they are just becoming smaller and smaller. But how far can you take this exercise, at least in theory? Can you continue cutting the aluminum foil into halves forever, making smaller and smaller pieces? Or is there some limit, some absolute smallest piece of aluminum foil? (Thought experiments like this—and the conclusions based on them—were debated as far back as the fifth century BC.)
The modern atomic theory, proposed about 1803 by the English chemist John Dalton (Figure \(\PageIndex{1}\)), is a fundamental concept that states that all elements are composed of atoms. Previously, we defined an atom as the smallest part of an element that maintains the identity of that element. Individual atoms are extremely small; even the largest atom has an approximate diameter of only 5.4 × 10−10 m. With that size, it takes over 18 million of these atoms, lined up side by side, to equal the width of your little finger (about 1 cm).
 Figure \(\PageIndex{1}\) John Dalton was an English scientist who enunciated the modern atomic theory.
Figure \(\PageIndex{1}\) John Dalton was an English scientist who enunciated the modern atomic theory.
Most elements in their pure form exist as individual atoms. For example, a macroscopic chunk of iron (Fe) metal is composed, microscopically, of individual atoms. Some elements, however, exist as groups of atoms called molecules. Several important elements exist as two-atom combinations and are called diatomic molecules. In representing a diatomic molecule, we use the symbol of the element and include the subscript "2" to indicate that two atoms of that element are joined together. The elements that exist as diatomic molecules are hydrogen (H2), nitrogen (N2), fluorine (F2), oxygen (O2), iodine (I2), chlorine (Cl2) and bromine (Br2). A few other elements can exist as 3-atom molecules like ozone (O3) and 4-atom molecules like phosphorus (P4). The most common form of the element sulfur is composed of molecules that consist of eight atoms of sulfur; its molecular formula is S8 (Figure \(\PageIndex{2}\)).
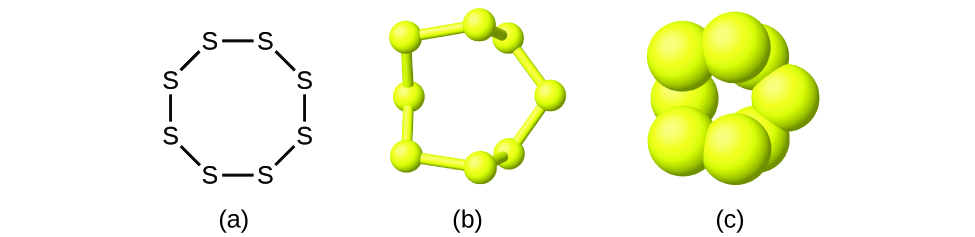 Figure \(\PageIndex{2}\): A molecule of sulfur is composed of eight sulfur atoms and is therefore written as S8. It can be represented as (a) a structural formula, (b) a ball-and-stick model, and (c) a space-filling model. Sulfur atoms are represented by yellow spheres.
Figure A shows eight sulfur atoms, symbolized with the letter S, that are bonded to each other to form an octagon. Figure B shows a 3-D, ball-and-stick model of the arrangement of the sulfur atoms. The shape is clearly not octagonal as it is represented in the structural formula. Figure C is a space-filling model that shows each sulfur atom is partially embedded into the sulfur atom it bonds with.
Figure \(\PageIndex{2}\): A molecule of sulfur is composed of eight sulfur atoms and is therefore written as S8. It can be represented as (a) a structural formula, (b) a ball-and-stick model, and (c) a space-filling model. Sulfur atoms are represented by yellow spheres.
Figure A shows eight sulfur atoms, symbolized with the letter S, that are bonded to each other to form an octagon. Figure B shows a 3-D, ball-and-stick model of the arrangement of the sulfur atoms. The shape is clearly not octagonal as it is represented in the structural formula. Figure C is a space-filling model that shows each sulfur atom is partially embedded into the sulfur atom it bonds with.
It is important to note that a subscript following a symbol and a number in front of a symbol do not represent the same thing; for example, H2 and 2H represent distinctly different species. H2 is a molecular formula; it represents a diatomic molecule of hydrogen, consisting of two atoms of the element that are chemically bonded together. The expression 2H, on the other hand, indicates two separate hydrogen atoms that are not combined as a unit. The expression 2H2 represents two molecules of diatomic hydrogen (Figure \(\PageIndex{3}\)).
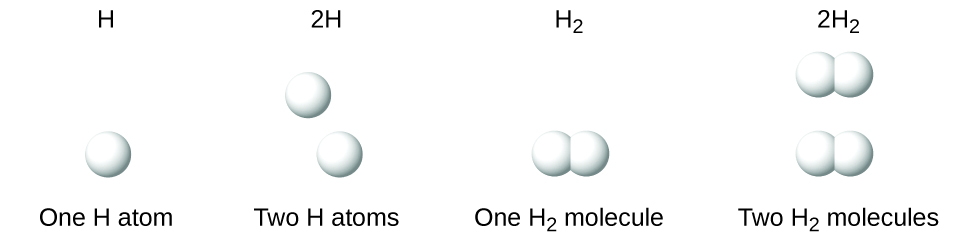 Figure \(\PageIndex{3}\): The symbols H, 2H, H2, and 2H2 represent very different entities.
This figure shows four diagrams. The diagram for H shows a single, white sphere and is labeled one H atom. The diagram for 2 H shows two white spheres that are not bonded together. It is labeled 2 H atoms. The diagram for H subscript 2 shows two white spheres bonded together. It is labeled one H subscript 2 molecule. The diagram for 2 H subscript 2 shows two sets of bonded, white spheres. It is labeled 2 H subscript 2 molecules.
Figure \(\PageIndex{3}\): The symbols H, 2H, H2, and 2H2 represent very different entities.
This figure shows four diagrams. The diagram for H shows a single, white sphere and is labeled one H atom. The diagram for 2 H shows two white spheres that are not bonded together. It is labeled 2 H atoms. The diagram for H subscript 2 shows two white spheres bonded together. It is labeled one H subscript 2 molecule. The diagram for 2 H subscript 2 shows two sets of bonded, white spheres. It is labeled 2 H subscript 2 molecules.
Write the chemical formula of the following elements:
- oxygen
- carbon
- potassium
Solution
Memorizing the diatomic molecules is worthwhile for our future endeavors. A mnemonic device to help in the memorization of the diatomic elements is as follows: Have No Fear Of Ice Cold Beer. (hydrogen, nitrogen, fluorine, oxygen, iodine, chlorine and bromine).
- Oxygen is one of the seven diatomic molecular elements. Its chemical formula is O2.
- Carbon is monatomic, hence its formula is C.
- Potassium is monatomic hence its formula is K.
Exercise \(\PageIndex{1}\)
Write the chemical formula of the following elements:
- hydrogen
- nitrogen
- neon
- Answer a
-
Hydrogen is one of the seven diatomic molecular elements. Its chemical formula is H2.
- Answer b
-
Nitrogen is one of the seven diatomic molecular elements. Its chemical formula is N2.
- Answer c
-
Neon is a monatomic element, hence its formula is Ne.
Dalton’s ideas are called the modern atomic theory because the concept of atoms is very old. The Greek philosophers Leucippus and Democritus originally introduced atomic concepts in the fifth century BC. (The word atom comes from the Greek word atomos, which means “indivisible” or “uncuttable.”) Dalton had something that the ancient Greek philosophers didn’t have, however; he had experimental evidence, such as the formulas of simple chemicals and the behavior of gases. In the 150 years or so before Dalton, natural philosophy had been maturing into modern science, and the scientific method was being used to study nature. So when Dalton announced a modern atomic theory, he was proposing a fundamental theory to describe many previous observations of the natural world; he was not just participating in a philosophical discussion.
Concept Review Exercises
- What is the modern atomic theory?
- What are atoms?
Answers
- The modern atomic theory states that all matter is composed of atoms.
- Atoms are the smallest parts of an element that maintain the identity of that element.
Key Takeaways
- Atoms are the ultimate building blocks of all matter.
- The modern atomic theory establishes the concepts of atoms and how they compose matter.
- The diatomic elements are hydrogen, nitrogen, fluorine, oxygen, iodine, chlorine and bromine.

