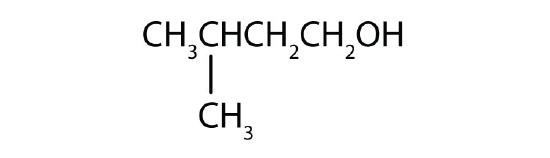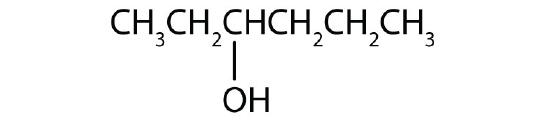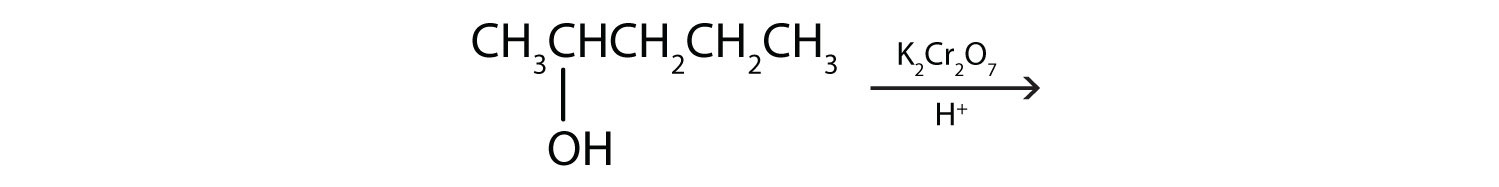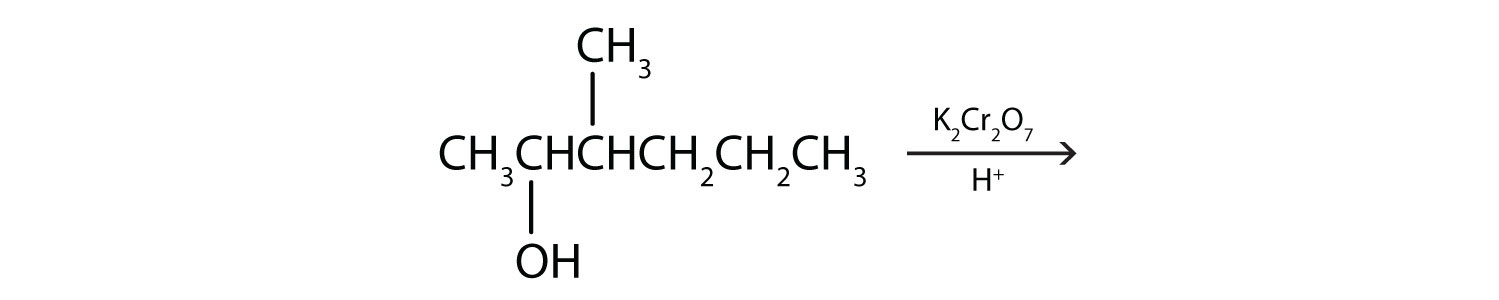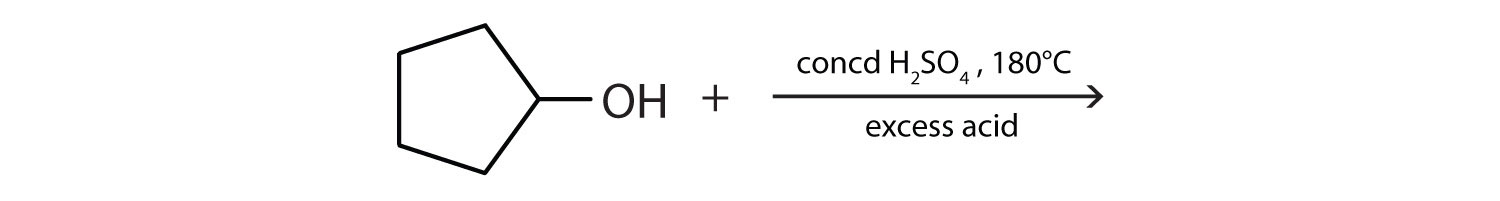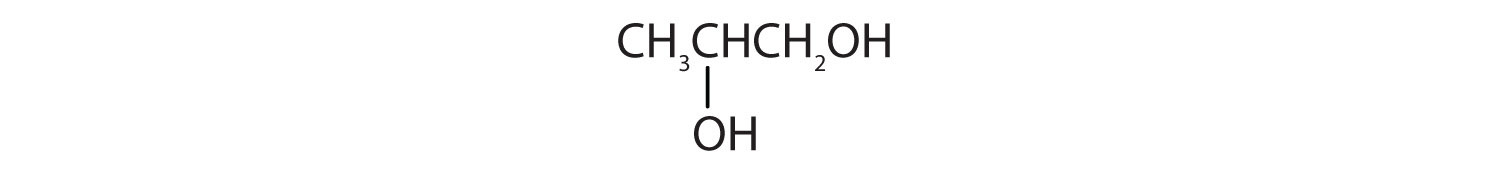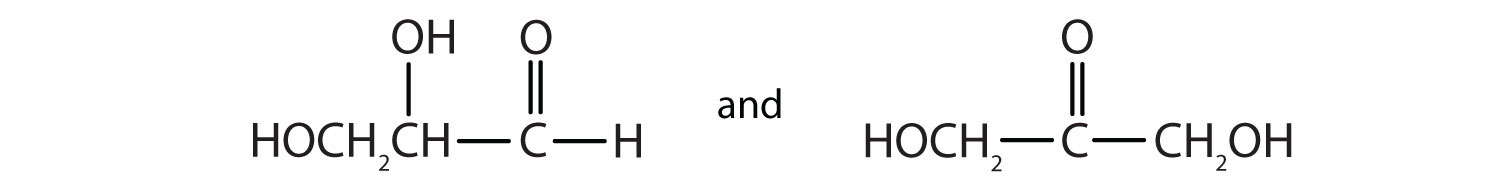13.13: Organic Compounds of Oxygen (Exercises)
- Page ID
- 15329
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Concept Review Exercises
- What is the functional group of an alkene? An alkyne?
- Does CH3CH2CH2CH2CH2CH2CH2CH2CH2CH2CH3 have a functional group? Explain.
Answers
- carbon-to-carbon double bond; carbon-to-carbon triple bond
- No; it has nothing but carbon and hydrogen atoms and all single bonds.
Exercises
- What is the functional group of 1-butanol (CH3CH2CH2CH2OH)?
- What is the functional group of butyl bromide, CH3CH2CH2CH2Br?
Answer
- OH
Concept Review Exercises
- Is isobutyl alcohol primary, secondary, or tertiary? Explain.
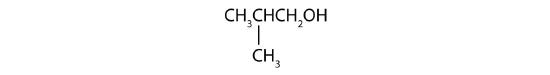
- What is the LCC in 2-ethyl-1-hexanol? What is taken as the LCC in naming the compound? Explain.
Answers
- primary; the carbon atom bearing the OH group is attached to only one other carbon atom
- 7 carbon atoms; the 6-atom chain includes the carbon atom bearing the OH group
Exercises
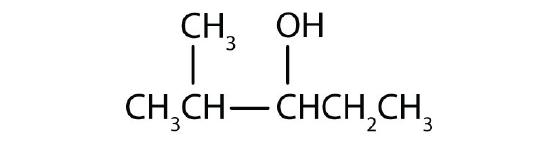
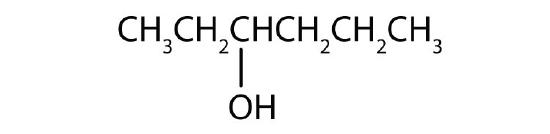
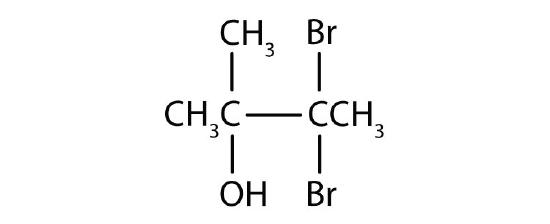
- Name each alcohol and classify it as primary, secondary, or tertiary.
- CH3CH2CH2CH2CH2CH2OH
-
-
- Name each alcohol and classify it as primary, secondary, or tertiary.
-
- Draw the structure for each alcohol.
- 3-hexanol
- 3,3-dimethyl-2-butanol
- cyclobutanol
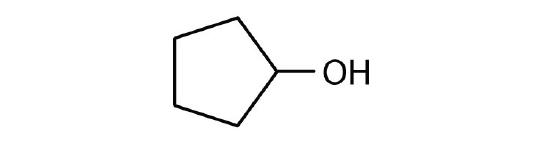
- Draw the structure for each alcohol.
- cyclopentanol
- 4-methyl-2-hexanol
- 4,5-dimethyl-3-heptanol
Answers
-
- 1-hexanol; primary
- 3-hexanol; secondary
- 3,3-dibromo-2-methyl-2-butanol; tertiary
Concept Review Exercises
- Why is ethanol more soluble in water than 1-hexanol?
- Why does 1-butanol have a lower boiling point than 1-hexanol?
Answers
- Ethanol has an OH group and only 2 carbon atoms; 1-hexanol has one OH group for 6 carbon atoms and is thus more like a (nonpolar) hydrocarbon than ethanol is.
- The molar mass of 1-hexanol is greater than that of 1-butanol.
Exercises
Answer the following exercises without consulting tables in the text.
- Arrange these alcohols in order of increasing boiling point: ethanol, methanol, and 1-propanol.
- Which has the higher boiling point—butane or 1-propanol?
- Arrange these alcohols in order of increasing solubility in water: 1-butanol, methanol, and 1-octanol.
- Arrange these compounds in order of increasing solubility in water: 1-butanol, ethanol, and pentane.
Answers
- methanol < ethanol < 1-propanol
- 1-octanol < 1-butanol < methanol
Concept Review Exercises
- Why is methanol more toxic than ethanol?
- How does rubbing alcohol cool a feverish patient?
Answers
- Methanol is oxidized to formaldehyde, which destroys tissue; ethanol is oxidized to acetaldehyde and then acetic acid, a normal metabolite.
- Evaporation removes heat.
Exercises
- From what alkene is ethanol made? Draw its condensed structural formula.
- Can methanol be made from an alkene? Explain.
Answer
- ethylene; CH2=CH2
14.5: Reactions of Alcohols
Conceptual Questions
-
In a reaction, compound W with the molecular formula C4H10O is converted to compound X with the formula C4H8O. Is W oxidized, reduced, dehydrated, or none of these? Explain.
-
In a reaction, 2 mol of compound Y with the molecular formula C4H10O is converted to 1 mol of compound Z with the formula C8H18O. Is Y oxidized, reduced, or neither? Explain.
Answers
-
oxidized; H is removed
-
neither; water is removed
Exercises
-
Name the three major types of chemical reactions of alcohols.
-
Why do tertiary alcohols not undergo oxidation? Can a tertiary alcohol undergo dehydration?
-
Draw the structure of the product for each reaction.
-
-
Draw the structure of the product for each reaction.
-
-
Write an equation for the dehydration of 2-propanol to yield each compound type.
- an alkene
- an ether
-
Draw the structure of the alkene formed by the dehydration of cyclohexanol.
Answers
-
dehydration, oxidation, and esterification
-
-
\(\mathrm{CH_3CHOHCH_3\underset{180^\circ C,\: excess\: acid}{\xrightarrow{conc\: H_2SO_4}} CH_3COCH_3+H_2O}\)
-
\(\mathrm{2CH_3CHOHCH_3 \underset{180^\circ C,\: excess\: acid}{\xrightarrow{conc\: H_2SO_4}}(CH_3)_2CHOCH(CH_3)_2+H_2O}\)
-
14.6: Glycols and Glycerol
Concept Review Exercises
- In the oxidation of propylene glycol to pyruvic acid, what functional groups in the reactant are involved? What new functional groups appear in the product?
- Oxalate ion is formed by the oxidation of ethylene glycol. In what kind of reaction is the oxalate ion involved?
Answers
- two OH groups; a ketone group and a carboxylic acid group
- precipitation
Exercises
- What is a glycol?
- Why is ethylene glycol so much more toxic to humans than propylene glycol?
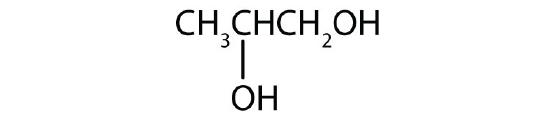
- Draw the structure for each compound.
- 1,5-pentanediol
- propylene glycol
- Draw the structure for each compound.
- 1,3-hexandiol
- glycerol
Answers
- an alcohol with two OH groups on adjacent carbon atoms
-
- HOCH2CH2CH2CH2CH2OH
-
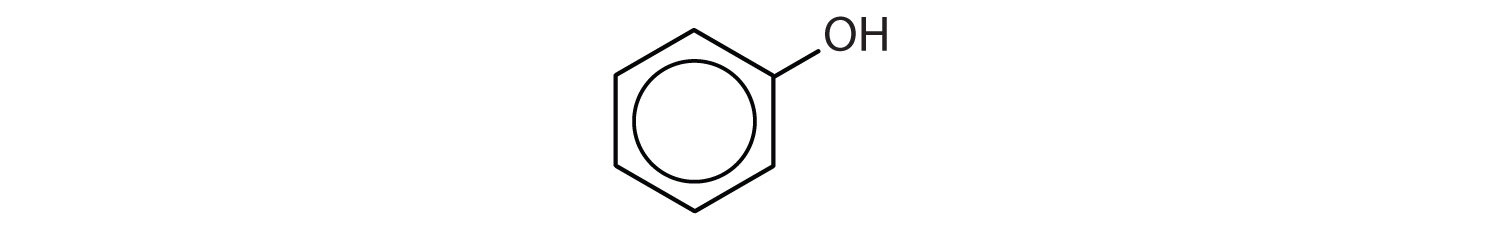
14.7: Phenols
Concept Review Exercises
- How do phenols differ from alcohols in terms of structure and properties?
- How do phenols differ in properties from aromatic hydrocarbons?
Answers
- Phenols have an OH group attached directly to an aromatic ring. Phenols are weakly acidic.
- Phenols have an OH group and are somewhat soluble in water.
Exercises
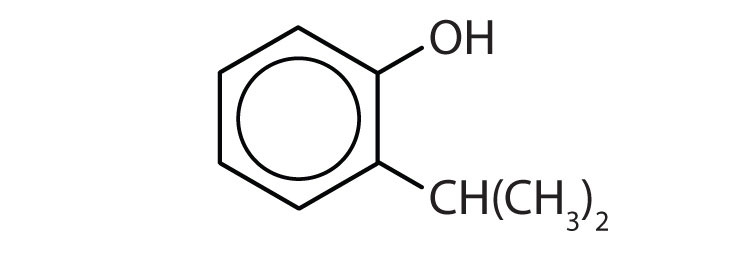
- Name each compound.
-
- Name each compound.
-
- Draw the structure for each compound.
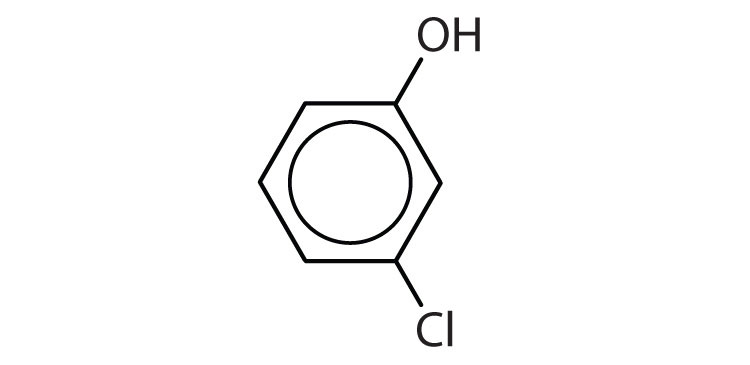
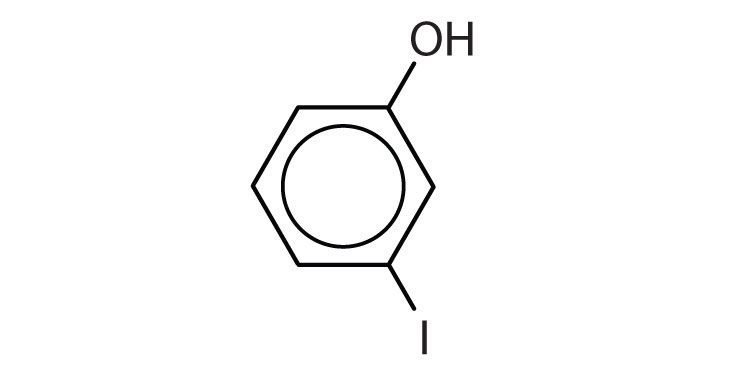
- m-iodophenol
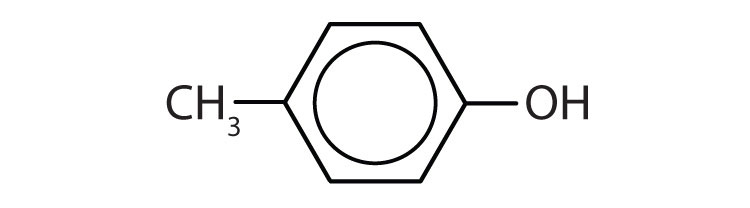
- p-methylphenol (p-cresol)
- Draw the structure for each compound.
- 2,4,6-trinitrophenol (picric acid)
- 3,5-diethylphenol
Answers
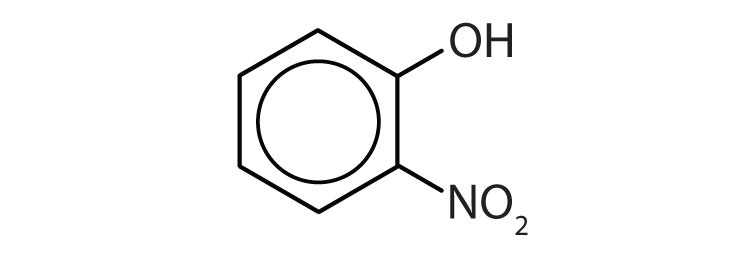
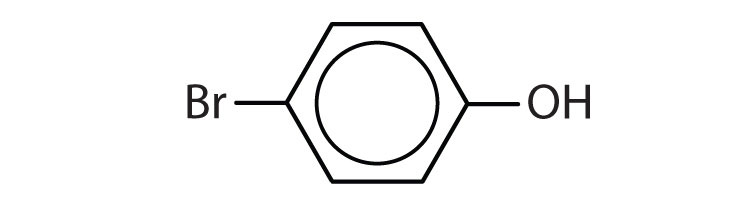
-
- o-nitrophenol
- p-bromophenol
14.8: Ethers
Concept Review Exercises
- Why does diethyl ether (CH3CH2OCH2CH3) have a much lower boiling point than 1-butanol (CH3CH2CH2CH2OH)?
- Which is more soluble in water—ethyl methyl ether (CH3CH2OCH3) or 1-butanol (CH3CH2CH2CH2OH)? Explain.
Answers
- Diethyl ether has no intermolecular hydrogen bonding because there is no OH group; 1-butanol has an OH and engages in intermolecular hydrogen bonding.
- Ethyl methyl ether (three carbon atoms, one oxygen atom) is more soluble in water than 1-butanol (four carbon atoms, one oxygen atom), even though both can engage in hydrogen bonding with water.
Exercises
- How can ethanol give two different products when heated with sulfuric acid? Name these products.
- Which of these ethers is isomeric with ethanol—CH3CH2OCH2CH3, CH3OCH2CH3, or CH3OCH3?
- Name each compound.
- CH3OCH2CH2CH3
-
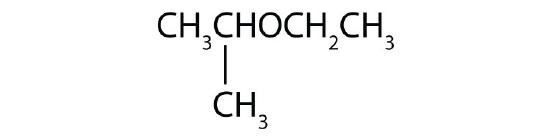
- Name each compound.
- CH3CH2CH2CH2OCH3
- CH3CH2OCH2CH2CH3
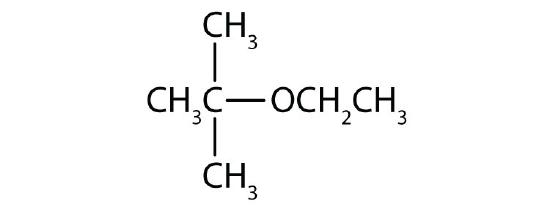
- Draw the structure for each compound.
- methyl ethyl ether
- tert-butyl ethyl ether
- Draw the structure for each compound.
- diisopropyl ether
- cyclopropyl propyl ether
Answers
- Intramolecular (both the H and the OH come from the same molecule) dehydration gives ethylene; intermolecular (the H comes from one molecule and the OH comes from another molecule) dehydration gives diethyl ether.
-
- methyl propyl ether
- ethyl isopropyl ether
-
- CH3OCH2CH3
-
14.9: Aldehydes and Ketones- Structure and Names
Concept Review Exercises
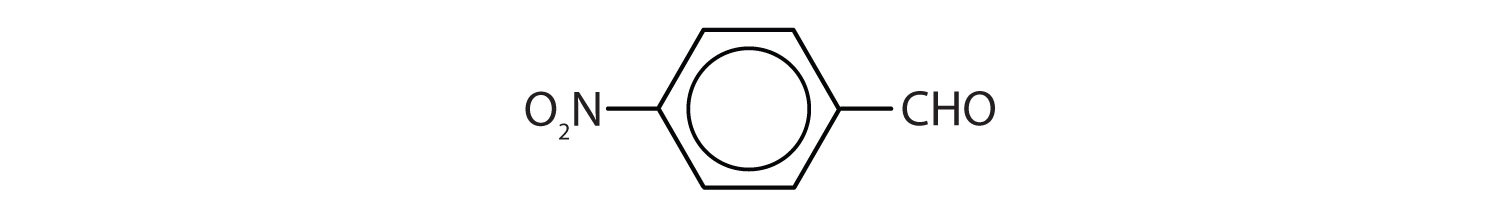
- Give the structure and IUPAC name for the compound that has the common name m-bromobenzaldehyde.
- Give the IUPAC name for glyceraldehyde, (HOCH2CHOHCHO). (Hint: as a substituent, the OH group is named hydroxy.)
Answers
-
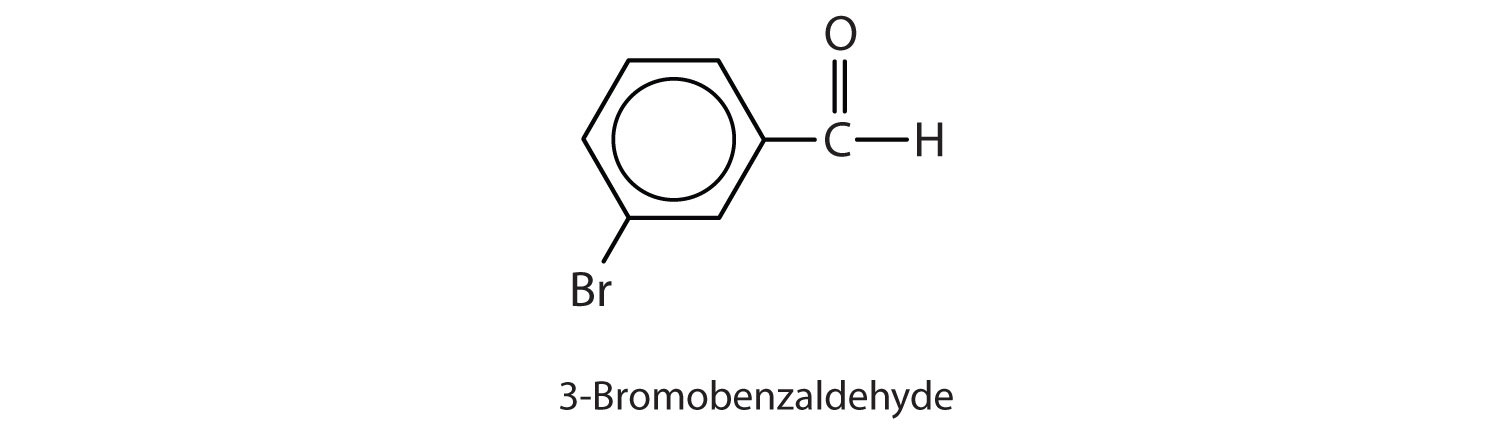
3-bromobenzaldehyde
-
2,3-dihydroxypropanal
Answers
- The stem names of aldehydes and ketones are derived from those of the parent alkanes, defined by the longest continuous chain (LCC) of carbon atoms that contains the functional group.
- For an aldehyde, drop the -e from the alkane name and add the ending -al. Methanal is the IUPAC name for formaldehyde, and ethanal is the name for acetaldehyde.
- For a ketone, drop the -e from the alkane name and add the ending -one. Propanone is the IUPAC name for acetone, and butanone is the name for ethyl methyl ketone.
- To indicate the position of a substituent on an aldehyde, the carbonyl carbon atom is always considered to be C1; it is unnecessary to designate this group by number.
- To indicate the position of a substituent on a ketone, number the chain in the manner that gives the carbonyl carbon atom the lowest possible number. In cyclic ketones, it is understood that the carbonyl carbon atom is C1.
-
Give the structure and IUPAC name for the compound that has the common name m-bromobenzaldehyde.
-
Give the IUPAC name for glyceraldehyde, (HOCH2CHOHCHO). (Hint: as a substituent, the OH group is named hydroxy.)
-

3-bromobenzaldehyde
-
2,3-dihydroxypropanal
-
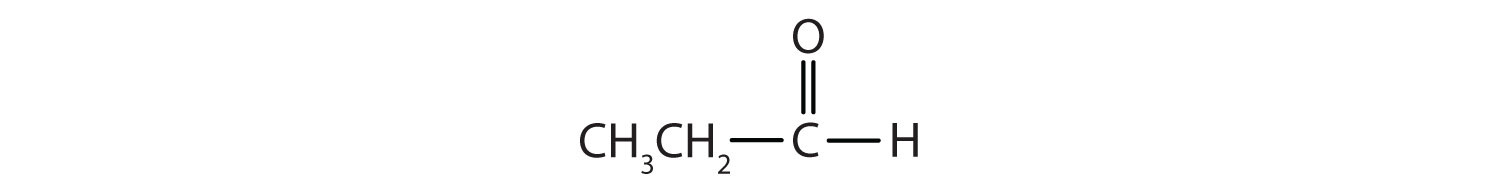
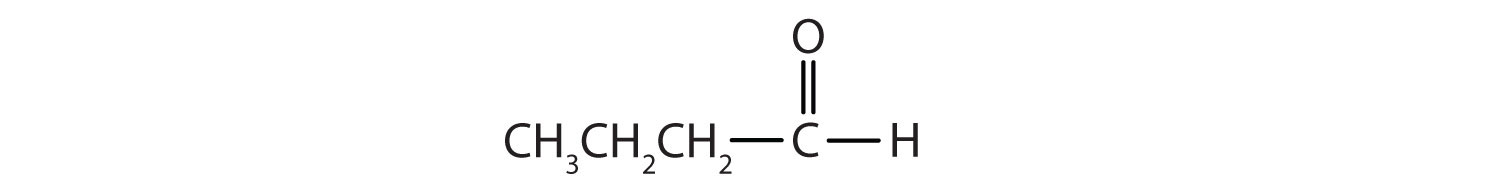
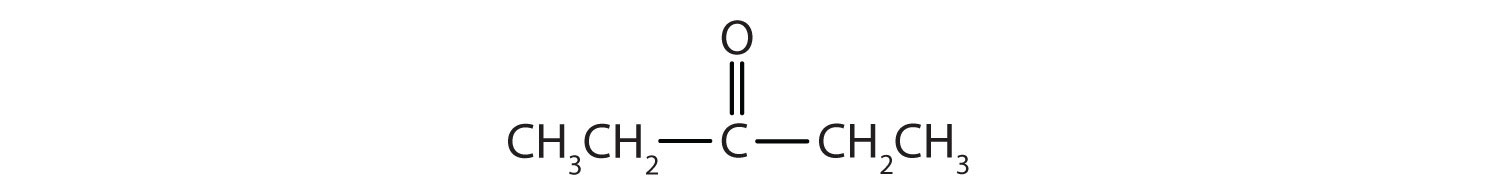
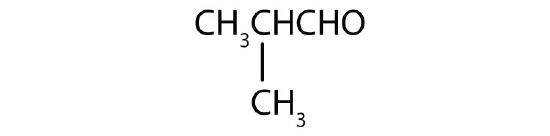
Name each compound.
-
-
Name each compound.
-
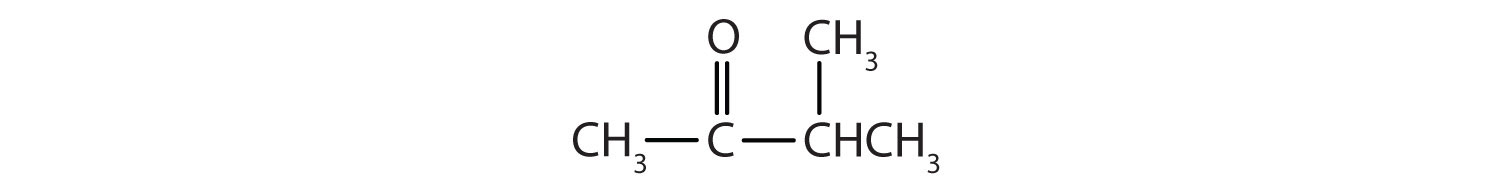
- CH3CH2CH2CH2CH2CHO
-
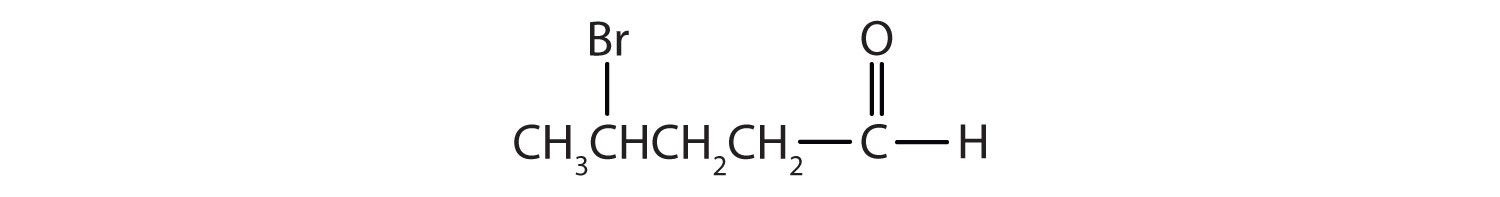
-
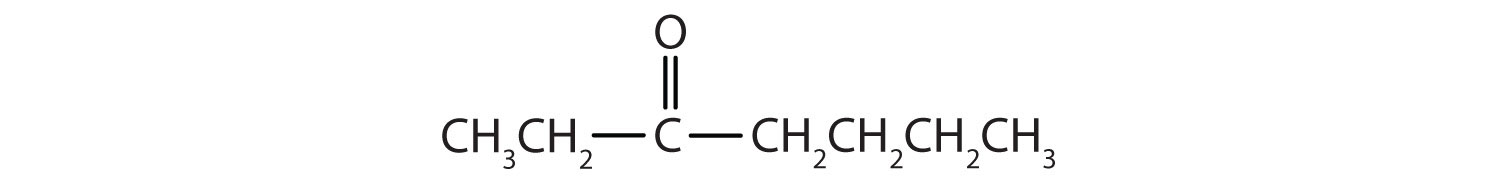
-
-
Draw the structure for each compound.
- butyraldehyde
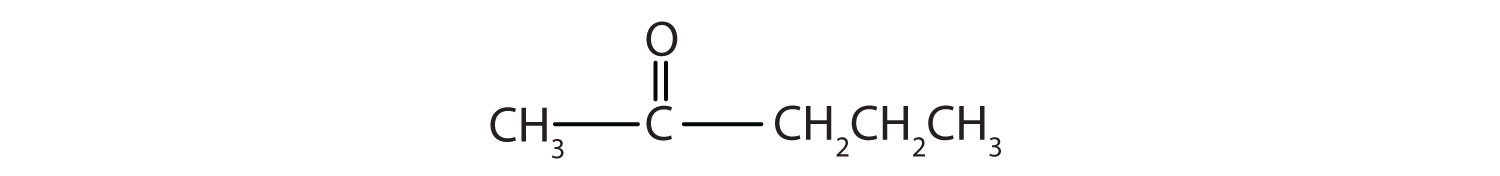
- 2-hexanone
- p-nitrobenzaldehyde
-
Draw the structure for each compound.
- 5-ethyloctanal
- 2-chloropropanal
- 2-hydroxy-3-pentanone
-
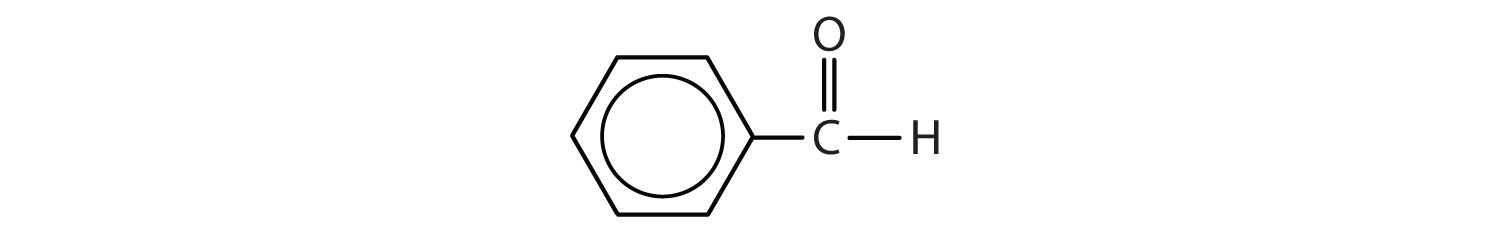
- propanal or propionaldehyde
- butanal or butyraldehyde
- 3-pentanone or diethyl ketone
- benzaldehyde
-
- CH3CH2CH2CHO
-
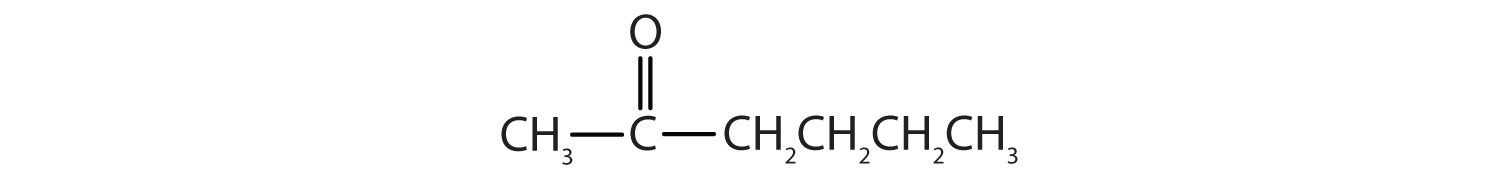
-
14.10: Properties of Aldehydes and Ketones
Concept Review Exercises
-
What feature of their structure makes aldehydes easier to oxidize than ketones?
-
How does the carbon-to-oxygen bond of aldehydes and ketones differ from the carbon-to-carbon bond of alkenes?
Answers
-
the H on the carbonyl carbon atom
-
The carbon-to-oxygen double bond is polar; the carbon-to-carbon double bond is nonpolar.
Exercises
-
Which compound in each pair has the higher boiling point?
- acetone or 2-propanol
- dimethyl ether or acetaldehyde
-
Which compound in each pair has the higher boiling point?
- butanal or 1-butanol
- acetone or isobutane
-
Draw the structure of the alcohol that could be oxidized to each compound.
- cyclohexanone
- 2-methyl-1-propanal
-
Draw the structure of the alcohol that could be oxidized to each compound.
- 2-pentanone
- o-methylbenzaldehyde
-
Acetaldehyde is treated with each substance.
- Ag+(aq)—What inorganic product, if any, is formed?
- K2Cr2O7 in an acid solution—What organic product, if any, is formed?
-
Acetone is treated with each substance.
- Ag+(aq) —What inorganic product, if any, is formed?
- K2Cr2O7 in an acid solution—What organic product, if any, is formed?
Answers
-
- 2-propanol
- acetaldehyde
-
- silver metal (Ag)
- acetic acid (CH3COOH)
14.11: Organic Sulfur Compounds
Concept Review Exercises
- What is the functional group of a thiol? Write the condensed structural formula for ethanethiol (ethyl mercaptan).
- What is the functional group of a disulfide? Write the condensed structural formula for dipropyl disulfide.
Answers
- SH; CH3CH2SH
- –S–S–; CH3CH2CH2SSCH2CH2CH3
Exercises
- A common natural gas odorant is tert-butyl mercaptan. What is its condensed structural formula?
- Write the equation for the oxidation of ethanethiol to diethyl disulfide.
Answer
- (CH3)3CSH
Extra Exercises
-
Describe two ways that ethanol can be prepared. Which method is used to produce alcoholic beverages?
-
Give the structure of the alkene from which isopropyl alcohol is made by reaction with water in an acidic solution.
-
Ethanol is used as a solvent for some drugs that are not soluble in water. Why is methanol not used in medicines?
-
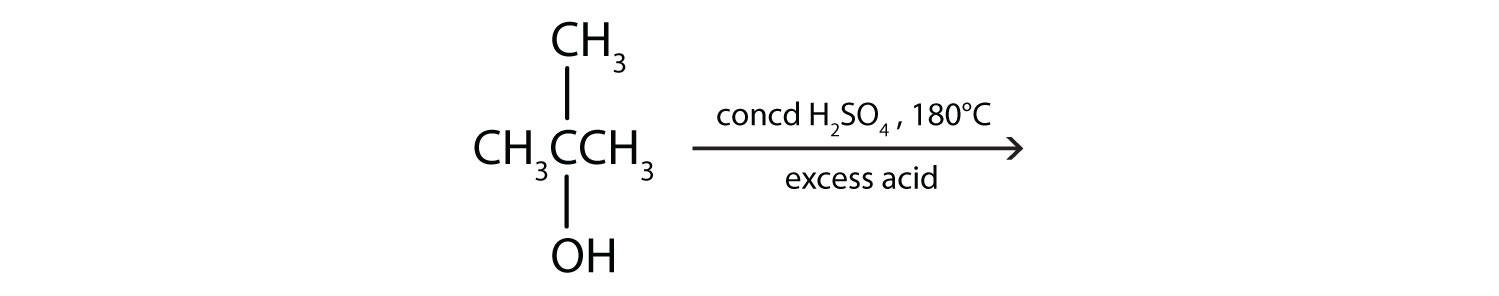
Give the structure of the alkene that is made from tert-butyl alcohol [(CH3)3COH] by reaction with water in an acidic solution.
-
Classify each conversion as oxidation, dehydration, or hydration (only the organic starting material and product are shown):
- CH3OH → HCHO
- CH3CHOHCH3 → CH3CH=CH2
- CH2=CHCH2CH3 → CH3CHOHCH2CH3
-
Classify each conversion as oxidation, dehydration, or hydration (only the organic starting material and product are shown.):
- CH3CHOHCH3 → CH3COCH3
- HOOCCH=CHCOOH → HOOCCH2CHOHCOOH
- 2 CH3OH → CH3OCH3
-
Why is methanol so much more toxic to humans than ethanol?
-
Each of the four isomeric butyl alcohols is treated with potassium dichromate (K2Cr2O7) in acid. Give the product (if any) expected from each reaction.
-
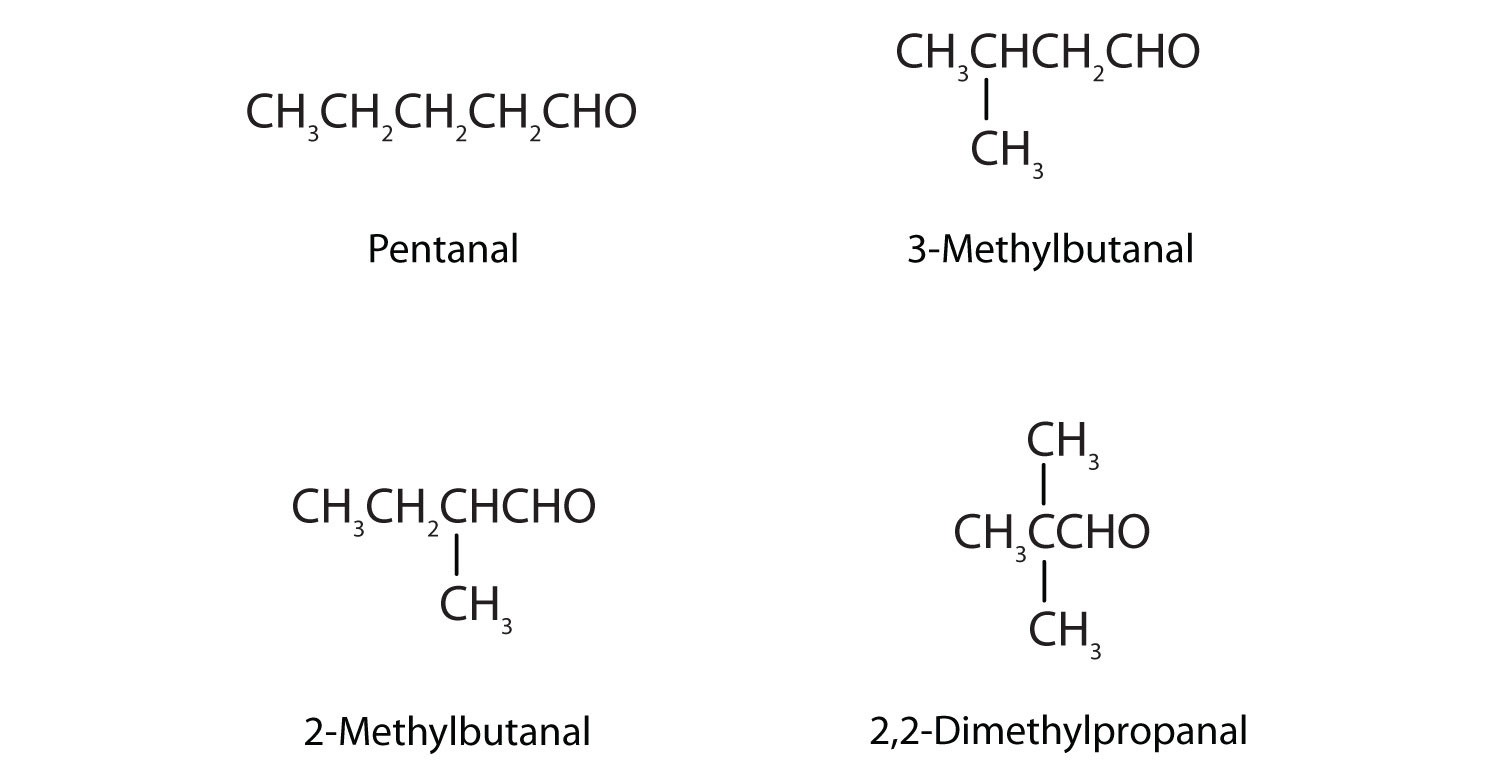
Draw the structures and give IUPAC names for the four isomeric aldehydes having the formula C5H10O.
-
Write an equation for the reaction of phenol with aqueous NaOH.
-
Write an equation for the ionization of phenol in water.
-
Draw the structures and give the common and IUPAC names for the three isomeric ketones having the formula C5H10O.
-
As we shall see in Chapter 16 "Carbohydrates", 2,3-dihydroxypropanal and 1,3-dihydroxyacetone are important carbohydrates. Draw their structures.
-
Glutaraldehyde (pentanedial) is a germicide that is replacing formaldehyde as a sterilizing agent. It is less irritating to the eyes, the nose, and skin. Write the condensed structural formula of glutaraldehyde.
-
Why does the oxidation of isopropyl alcohol give a ketone, whereas the oxidation of isobutyl alcohol gives an aldehyde?
-
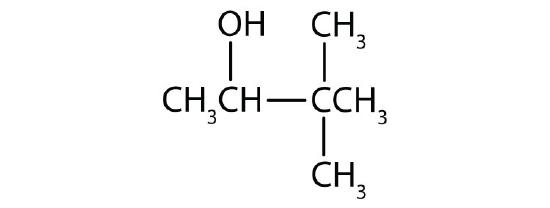
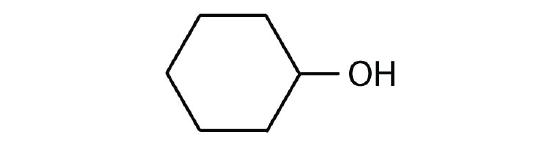
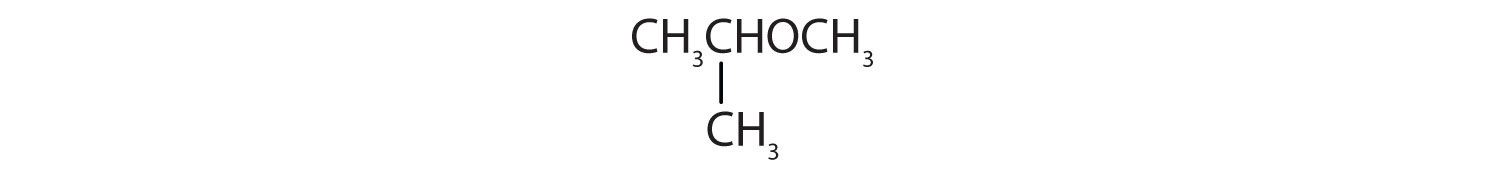
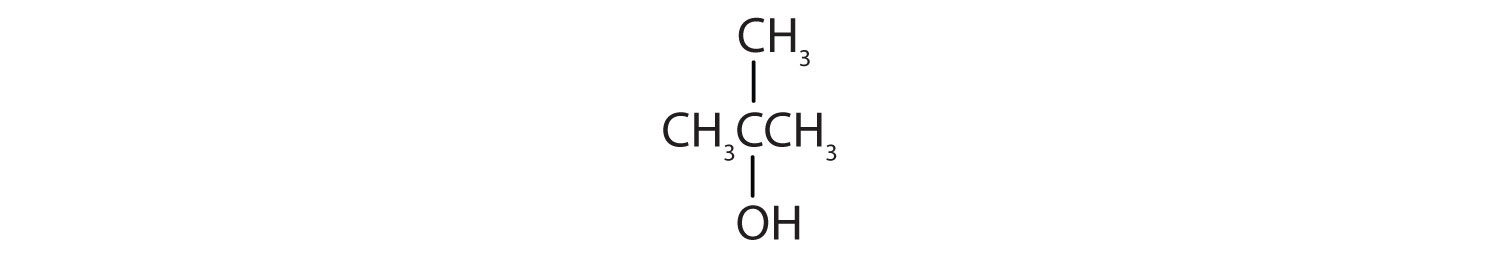
Identify each compound as an alcohol, a phenol, or an ether. Classify any alcohols as primary (1°), secondary (2°), or tertiary (3°).
- CH3CH2CH2OH
-
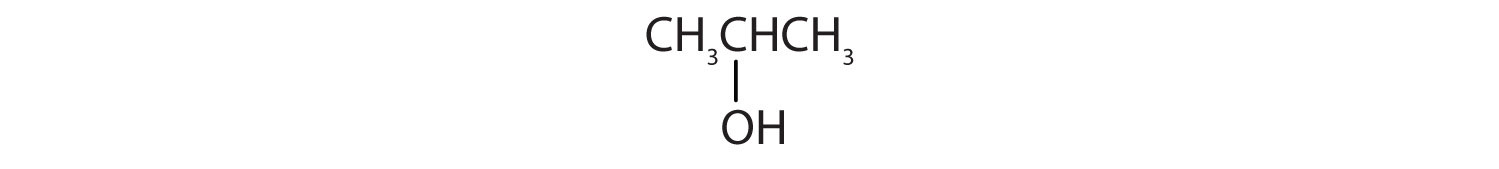
-
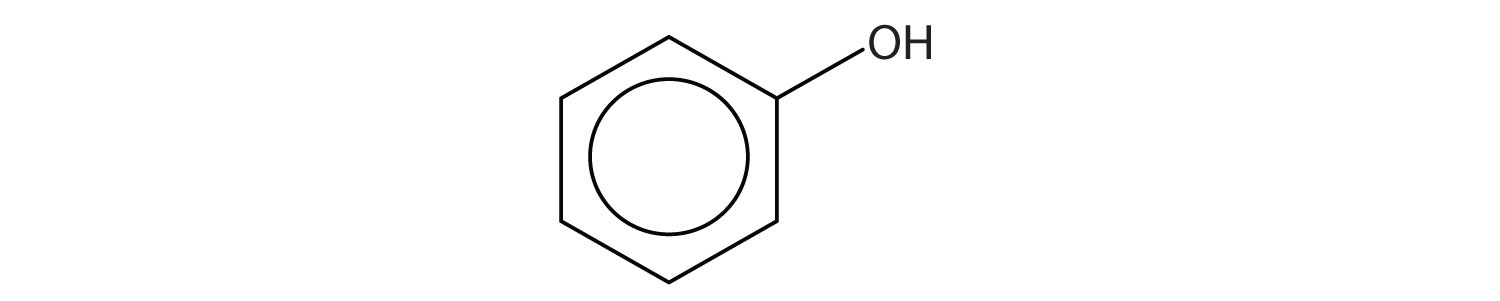
-
-
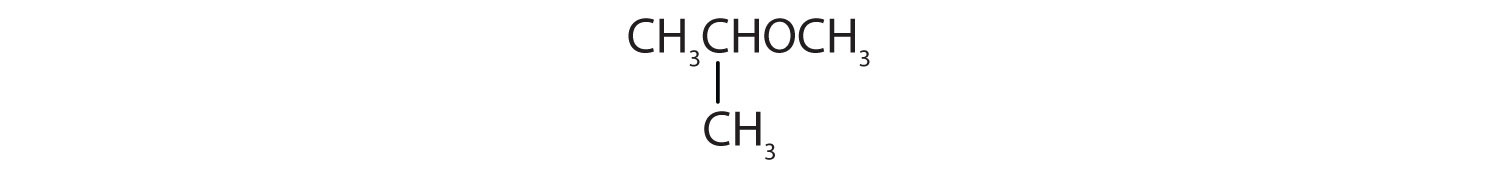
Identify each compound as an alcohol, a phenol, or an ether. Classify any alcohols as primary, secondary, or tertiary.
- CH3CH2OCH2CH3
-
-

-
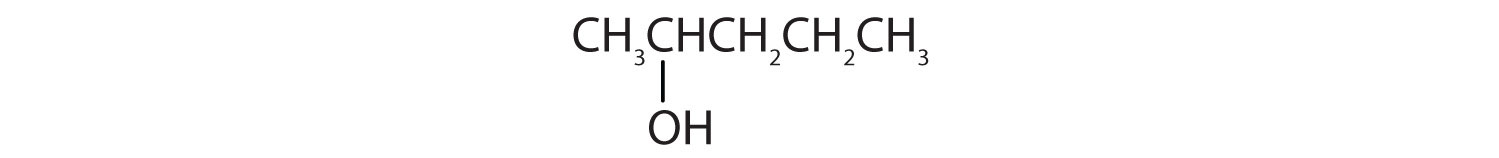
-
Tell whether each compound forms an acidic, a basic, or a neutral solution in water.
-
-
When water is added to ethylene in the presence of an acid catalyst, only one product—ethanol—is possible. However, when water is added to propylene, two products are possible—1-propanol and 2-propanol—but only 2-propanol is formed. In 1870, the Russian chemist Vladimir V. Markovnikov proposed a rule to predict the products of such reactions: Considering water to be HOH, the hydrogen atom of water goes on the carbon atom (of the two involved in the double bond) that has the most hydrogen atoms already bonded to it. The OH group goes on the carbon atom with fewer hydrogen atoms. Use Markovnikov’s rule to predict the product of the addition of water to each compound.
- 2-methylpropene
- 1-butene
- 2-methyl-1-pentene
- 2-methyl-2-pentene
-
Ethyl alcohol, like rubbing alcohol (isopropyl alcohol), is often used for sponge baths. What property of alcohols makes them useful for this purpose?
-
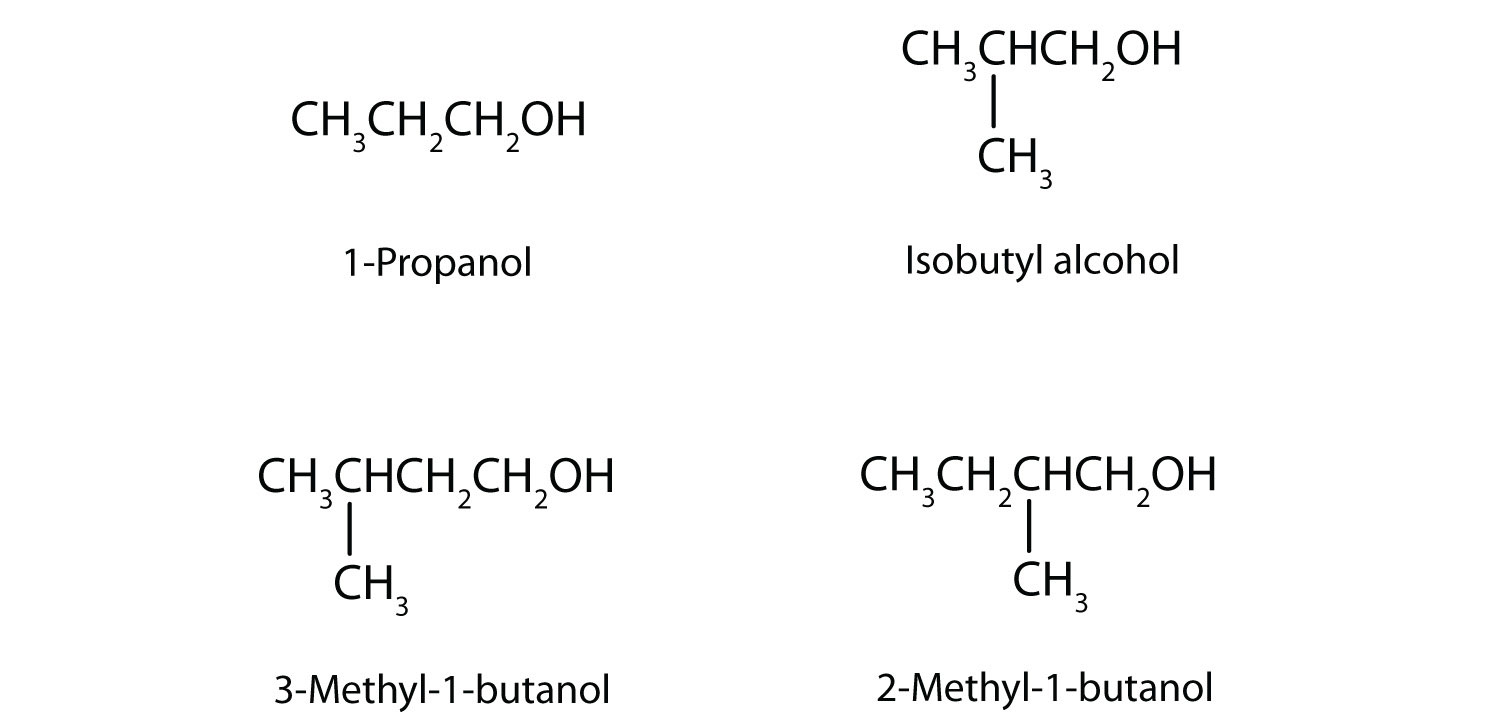
In addition to ethanol, the fermentation of grain produces other organic compounds collectively called fusel oils (FO). The four principal FO components are 1-propanol, isobutyl alcohol, 3-methyl-1-butanol, and 2-methyl-1-butanol. Draw a structure for each. (FO is quite toxic and accounts in part for hangovers.)
-
Draw and name the isomeric ethers that have the formula C5H12O.
-
Menthol is an ingredient in mentholated cough drops and nasal sprays. It produces a cooling, refreshing sensation when rubbed on the skin and so is used in shaving lotions and cosmetics. Thymol, the aromatic equivalent of menthol, is the flavoring constituent of thyme.
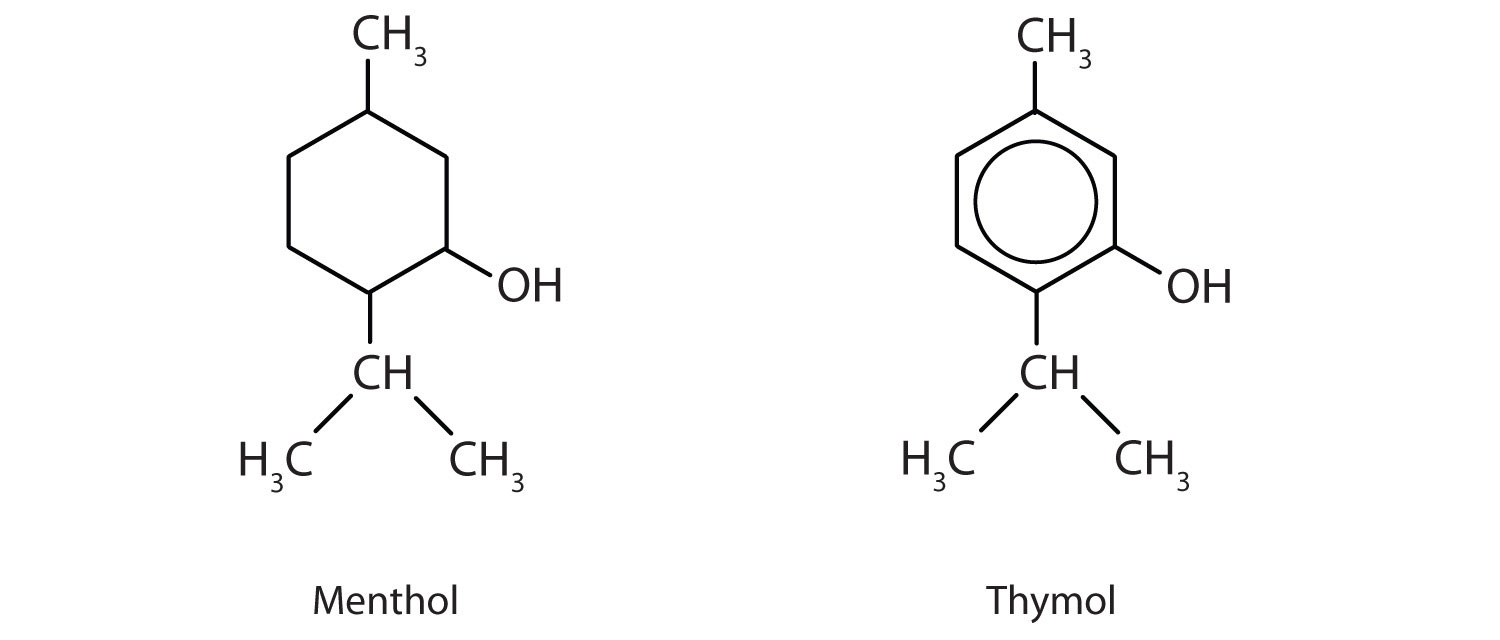
- To what class of compounds does each belong?
- Give an alternate name for thymol.
-
Write the equation for the production of ethanol by the addition of water to ethylene. How much ethanol can be made from 14.0 kg of ethylene?
-
Methanol is not particularly toxic to rats. If methanol were newly discovered and tested for toxicity in laboratory animals, what would you conclude about its safety for human consumption?
-
The amino acid cysteine has the formula HSCH2CH(NH2)COOH. What is the sulfur-containing functional group in the cysteine molecule?
-
The amino acid methionine has the formula CH3SCH2CH2CH(NH2)COOH. What functional groups are in methionine?
-
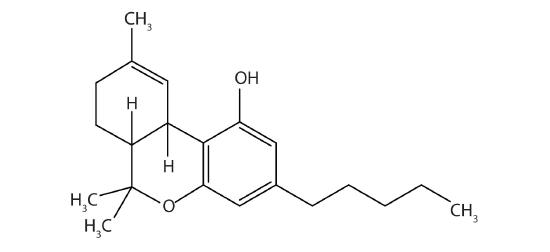
Tetrahydrocannabinol is the principal active ingredient in marijuana. What functional groups are present in this molecule?
Answers
-
addition of water to ethylene; fermentation (for beverages)
-
Methanol is too toxic.
-
- oxidation
- dehydration
- hydration
-
Methanol is oxidized in the body to toxic formaldehyde; ethanol is oxidized to the less toxic acetaldehyde.
-
C6H5OH + H2O → C6H5O− + H3O+
-
Isopropyl alcohol is a secondary alcohol, whereas isobutyl alcohol is a primary alcohol.
-
- ether
- tertiary alcohol
- phenol
- secondary alcohol
-
- tert-butyl alcohol
- 2-butanol
- 2-methyl-2-pentanol
- 2-methyl-2-pentanol
-
- menthol: alcohol; thymol: phenol
- 2-isopropyl-5-methylphenol
-
It might be ruled safe until tested on humans.
-
sulfide, amino, and carboxylic acid