14.11: Amines - Structures and Names
- Page ID
- 15342
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Identify the general structure for an amine.
- Identify the functional group for amines.
- Determine the structural feature that classifies amines as primary, secondary, or tertiary.
- Use nomenclature systems to name amines.
Amines are classified according to the number of carbon atoms bonded directly to the nitrogen atom. A primary (1°) amine has one alkyl (or aryl) group on the nitrogen atom, a secondary (2°) amine has two, and a tertiary (3°) amine has three (Figure \(\PageIndex{1}\)).
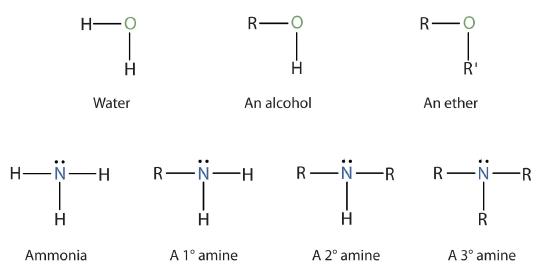 Figure \(\PageIndex{1}\): The Structure of Amines Compared to Water, an Alcohol, and an Ether
Figure \(\PageIndex{1}\): The Structure of Amines Compared to Water, an Alcohol, and an Ether
To classify alcohols, we look at the number of carbon atoms bonded to the carbon atom bearing the OH group, not the oxygen atom itself. Thus, although isopropylamine looks similar to isopropyl alcohol, the former is a primary amine, while the latter is a secondary alcohol.

The common names for simple aliphatic amines consist of an alphabetic list of alkyl groups attached to the nitrogen atom, followed by the suffix -amine. (Systematic names are often used by some chemists.) The amino group (NH2) is named as a substituent in more complicated amines, such as those that incorporate other functional groups or in which the alkyl groups cannot be simply named.
Name and classify each compound.
- CH3CH2CH2NH2
- CH3CH2NHCH2CH3
- CH3CH2CH2NHCH3
Solution
- There is only one alkyl group attached to the nitrogen atom, so the amine is primary. A group of three carbon atoms (a propyl group) is attached to the NH2 group through an end carbon atom, so the name is propylamine.
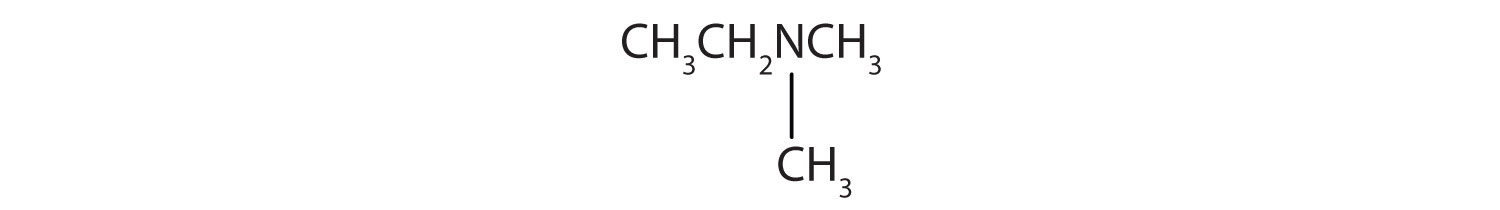
- There are two methyl groups and one ethyl group on the nitrogen atom. The compound is ethyldimethylamine, a tertiary amine.
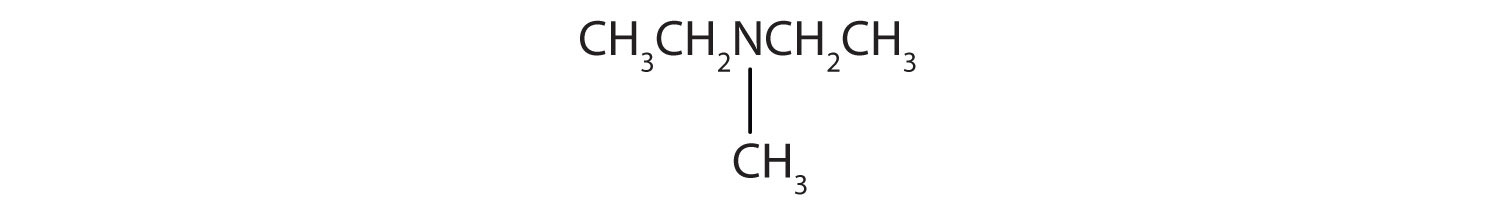
- There are two ethyl groups attached to the nitrogen atom; the amine is secondary, so the compound is diethylamine.
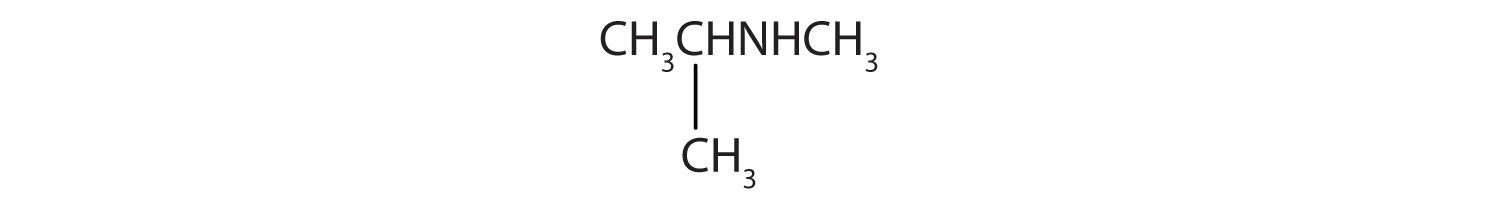
- The nitrogen atom has a methyl group and a propyl group, so the compound is methylpropylamine, a secondary amine.
Name and classify each compound.
- CH3CH2CH2CH2NH2
- CH3CH2CH2NHCH2CH2 CH3
Draw the structure for each compound and classify.
- isopropyldimethylamine
- dipropylamine
Solution
- The name indicates that there are an isopropyl group (in red) and two methyl groups (in green) attached to the nitrogen atom; the amine is tertiary.
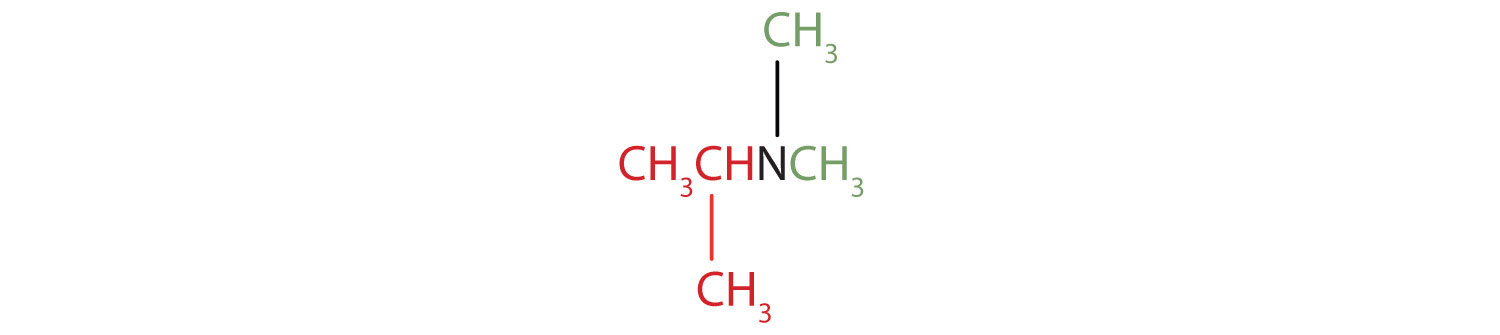
- The name indicates that there are two propyl groups attached to the nitrogen atom; the amine is secondary. (The third bond on the nitrogen atom goes to a hydrogen atom.) CH3CH2CH2NHCH2CH2CH3
Draw the structure for each compound and classify.
- ethylisopropylamine
- diethylpropylamine
The primary amine in which the nitrogen atom is attached directly to a benzene ring has a special name—aniline. Aryl amines are named as derivatives of aniline.

Name this compound.
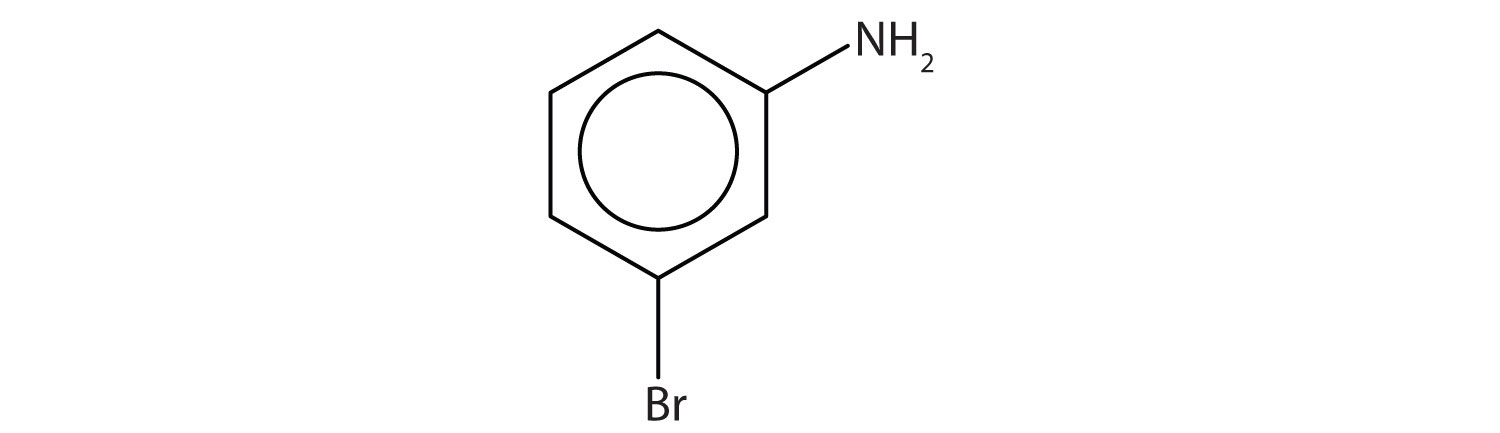
Solution
The benzene ring with an amino (NH2) group is aniline. The compound is named as a derivative of aniline: 3-bromoaniline or m-bromoaniline.
Name this compound.
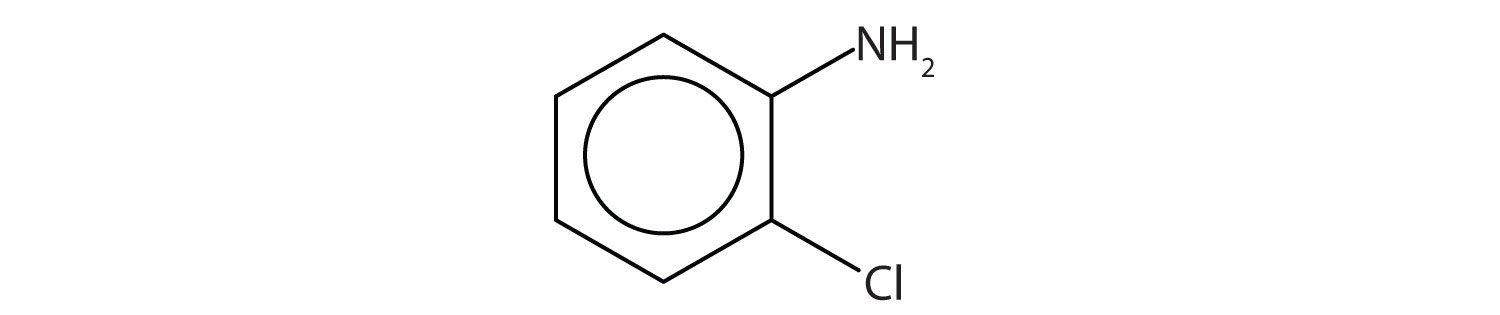
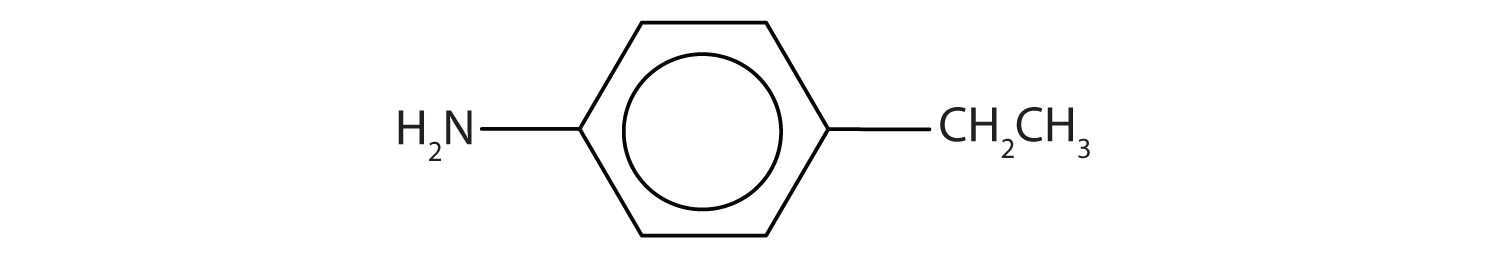
Draw the structure for p-ethylaniline and classify.
Solution
The compound is a derivative of aniline. It is a primary amine having an ethyl group located para to the amino (NH2) group.
Draw the structure for p-isopropylaniline and classify.
Draw the structure for 2-amino-3-methylpentane.
Solution
Always start with the parent compound: draw the pentane chain. Then attach a methyl group at the third carbon atom and an amino group at the second carbon atom.
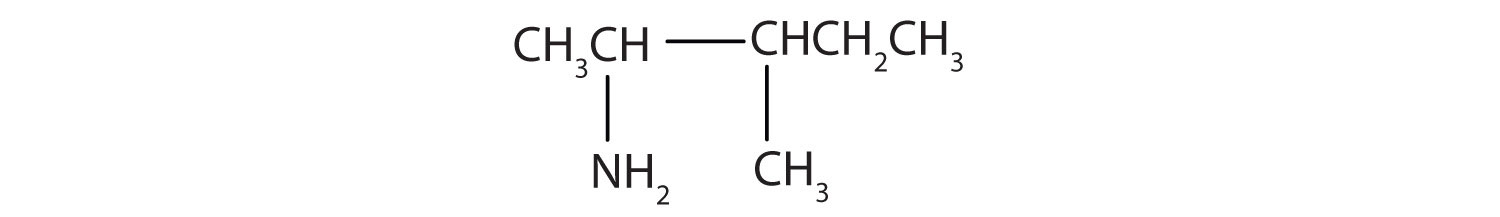
Draw the structure for 2-amino-3-ethyl-1-chloroheptane.
Ammonium (NH4+) ions, in which one or more hydrogen atoms are replaced with alkyl groups, are named in a manner analogous to that used for simple amines. The alkyl groups are named as substituents, and the parent species is regarded as the NH4+ ion. For example, CH3NH3+ is the methylammonium ion. The ion formed from aniline (C6H5NH3+) is called the anilinium ion.
Name each ion.
- CH3NH3+
- (CH3)2NH2+
- (CH3)3NH+
- (CH3)4N+
Solution
The ions have one, two, three, and four methyl (CH3) groups attached to a nitrogen atom. Their names are as follows:
- methylammonium ion
- dimethylammonium ion
- trimethylammonium ion
- tetramethylammonium ion
Name each ion.
- CH3CH2NH3+
- (CH3CH2)3NH+
- (CH3CH2CH2)2NH2+
- (CH3CH2CH2CH2)4N+
Summary
An amine is a derivative of ammonia in which one, two, or all three hydrogen atoms are replaced by hydrocarbon groups. The amine functional group is as follows:
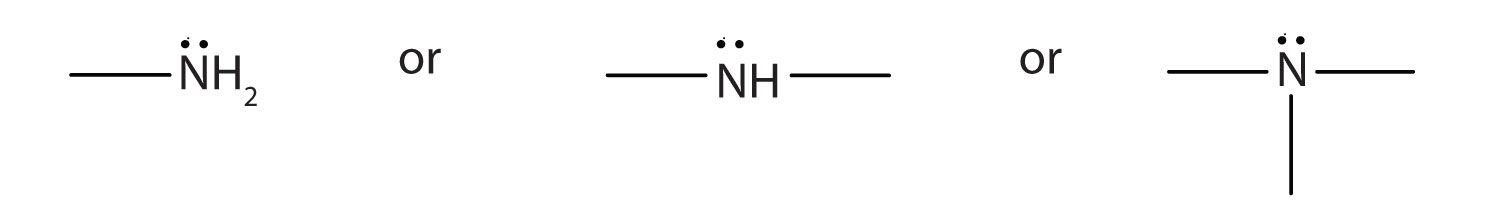
Amines are classified as primary, secondary, or tertiary by the number of hydrocarbon groups attached to the nitrogen atom. Amines are named by naming the alkyl groups attached to the nitrogen atom, followed by the suffix -amine.

