6.6: Basal Ganglia
- Page ID
- 70003
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Glossary terms
- Key Takeaways
- Test Yourself
The basal ganglia are a group of subcortical nuclei, meaning groups of neurons that lie below the cerebral cortex. The basal ganglia is comprised of the striatum, which consists of the caudate nucleus and the putamen, the globus pallidus, the subthalamic nucleus, and the substantia nigra The basal ganglia are intimately involved with various aspects of movement, such as voluntary motor activity, habit learning, and the selection of actions.
For voluntary motor behavior, the basal ganglia are involved in the initiation or suppression of behavior and can regulate movement through modulating activity in the thalamus and cortex. Despite being major structures involved in motor functions, none of the components of the basal ganglia directly send projections down the spinal cord. Rather, they communicate mostly within themselves before signaling through the motor cortex. While the connections between basal ganglia structures have been largely mapped out, the specific functions of each individual structure in isolation is not entirely clear.
Diseases of the basal ganglia include movement disorders such as Parkinson’s disease, dystonia, Huntington’s disease, and Tourette’s, as well as complex psychiatric disorders including addiction and obsessive-compulsive disorder (OCD).
Basal Ganglia Structures- Introduction
As we discuss the motor circuitry of the basal ganglia, it is important to understand that it is a loop. Activity from the cortex feeds into the basal ganglia, then through the thalamus, and then back to the cortex. The function of this loop of structures is to ultimately select and initiate willed movements.
The structures of the basal ganglia are briefly defined below.
Striatum– The striatum is made up of two components, the caudate nucleus and putamen. On a cellular level, the majority of neurons in the striatum are GABAergic cells.
Globus Pallidus– In dissection, the globus pallidus appears as a pale globe. It is subdivided into two components with functionally different connections. The globus pallidus internal segment (GPi) is located more medially and is movement promoting. On the other hand, the globus pallidus external segment (GPe) is located more laterally and is movement inhibiting. The output of the globus pallidus is only via inhibitory projections that are tonically active. This means that in the absence of any input, globus pallidus neurons will still inhibit their targets.
Subthalamic nucleus– The subthalamic nucleus is an output of the globus pallidus external segment. The output of the subthalamic nucleus are glutamatergic signals into the globus pallidus internal segment.
Substantia Nigra– The substantia nigra is a midbrain structure that appears darker in dissection due to heavy expression of neuromelanin across cells in these areas. It is the largest midbrain structure. The substantia nigra contains several dopamine expressing neurons that project into the striatum. Of clinical significance, these neurons experience selective neurodegeneration in Parkinson’s disease.

Basal Ganglia Input
The dorsal striatum receives both glutamatergic and dopaminergic inputs. The majority of information processed by the basal ganglia enters through the striatum, with projections coming from both cortical and limbic structures such as thalamus and amygdala. This input into the striatum is glutamatergic and therefore, excitatory. The striatum also receives dopaminergic projections from another basal ganglia structure, the substantia nigra, a communication route called the nigrostriatal pathway.
Dopamine plays an important role in basal ganglia function. Parkinson’s disease results when dopamine neurons in the substantia nigra degenerate and no longer send appropriate inputs to the striatum. Dopamine projections can have either excitatory or inhibitory effects in the striatum, depending on the type of metabotropic dopamine receptor the striatal neuron expresses. Dopamine action at a neuron that expresses the D1 receptor is excitatory. Dopamine action at a neuron that expresses the D2 receptor is inhibitory.


Basal Ganglia Output
The primary output region of the basal ganglia is the internal segment of the globus pallidus (GPi). This region sends inhibitory GABAergic projections to nuclei in the thalamus. This inhibitory output has a tonic, constant firing rate, which allows the basal ganglia output to both increase and decrease depending on the situation. The thalamus then projects back out to the cerebral cortex, primarily to motor areas.


Basal Ganglia Internal Processing
Direct Pathway
There are multiple connections within the basal ganglia structures as well. For motor control, there are two main circuits: the direct pathway and the indirect pathway. These circuits have opposing actions when activated by cortical neurons. The circuits are also modulated by dopamine release by the substantia nigra into the striatum. It is believed that the different control mechanisms allow a finely tuned balance between the direct and indirect circuits, which allows for refined control of movement.
The direct pathway begins with excitatory input from the cortex to the striatum (caudate and putamen). The striatum then sends inhibitory projections to the internal segment of the globus pallidus (GPi). The GPi then sends inhibitory output to the thalamus. The direct pathway initiates and facilitates voluntary movement.

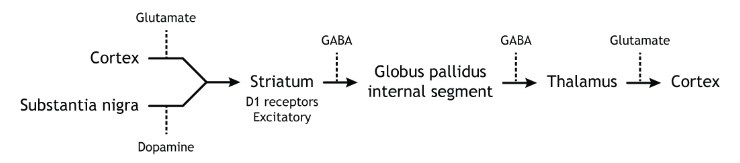
Activation of the Direct Pathway
To make a movement, there needs to be activation of the direct pathway.
When input from either the cortex or substantia nigra increases in intensity, the direct pathway is activated. The neurons in the striatum involved in the direct pathway express the D1 metabotropic dopamine receptor, and the activation of this receptor is excitatory. Therefore, projections from both the cortex and the substantia nigra activate the neurons in the striatum. The striatal neurons are inhibitory and release GABA onto the internal segment of the globus pallidus (GPi). As described above, the neurons in the GPi are inhibitory, releasing GABA onto the thalamus. Activation of the striatum neurons causes the release of GABA onto neurons in the GPi, inhibiting the GPi neurons. Inhibiting the GPi neurons releases their typical inhibition on the thalamus. Inhibition of an inhibitory region is called disinhibition. Therefore, the activation of the direct pathway results in increased output from the thalamus because it is disinhibited. Increased thalamic activity leads to increased cortical activity, and increased movement.
When at rest (not moving), there is not an increase in either glutamatergic or dopaminergic input into the striatum. As a result, there is no change in striatal activity to modulate activity of the GPi. The GPi will continue to tonically inhibit the thalamus, keeping cortical activity low and decreasing movement


Indirect pathway
The indirect pathway is a little more complex. Like the direct pathway, input into the basal ganglia arises from the cortex and substantia nigra, but there are more internal connections within the basal ganglia than what occurs in the direct pathway. Inhibitory neurons in the striatum involved in the indirect pathway project to the external segment of the globus pallidus (GPe). GABA-ergic neurons in the GPe project to the subthalamic nucleus, which then sends excitatory output to the GPi, which outputs to the thalamus.

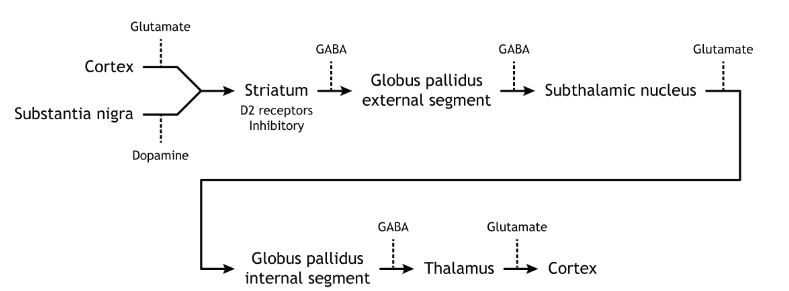
Activation of the Indirect Pathway
The indirect pathway is activated by excitatory cortical input, activating the inhibitory striatal neurons. This leads to inhibition of the GPe neurons, resulting in disinhibition of the excitatory neurons in the subthalamic nucleus. The excitatory output from the subthalamic nucleus to the GPi increases inhibition of the thalamus, leading to decreased thalamic output to the cortex.


Inhibition of the Indirect Pathway
The indirect pathway can be inhibited by dopamine release from the substantia nigra. The neurons in the striatum involved in the indirect pathway express the D2 metabotropic dopamine receptor. The activation of this receptor is inhibitory. If the indirect pathway is inhibited by dopamine projections from the substantia nigra, the inhibitory striatal neurons are inhibited. This leads to disinhibiton of the GPe neurons, resulting in inhibition of the excitatory neurons in the subthalamic nucleus. This decreased excitatory output to the GPi decreases inhibition of the thalamus, leading to increased thalamic output to the cortex.


Summary of Internal Processing
To put it all together, there is input to the striatum from two different locations: cortex (glutamate) and substantia nigra (dopamine).
- Cortical activation of the direct pathway leads to increased thalamic output
- Cortical activation of the indirect pathway leads to decreased thalamic output
- Substantia nigra activation (via D1) of the direct pathway leads to increased thalamic output
- Substantia nigra inhibition (via D2) of the indirect pathway leads to increased thalamic output
It is the combination of these pathways that allows for precise control of motor movement, balancing the excitatory direct pathway with the inhibitory indirect pathway.

Loops through the Basal Ganglia
There are multiple circuits that pass through the basal ganglia:
- The motor circuit, which plays a role in voluntary movement
- The oculomotor circuit, which plays a role in eye movement
- The associative circuit, which plays a role in executive functions like behavioral inhibition (preventing impulsive behaviors) planning and problem solving, and mediating socially appropriate behaviors
- The limbic or emotional circuit, which plays a role in the processing of emotion and reward.
Although the circuits each use different circuits within the basal ganglia, the general loop is the same: cortical input to the striatum leads to internal processing within the basal ganglia structures. Basal ganglia output projects from the pallidum to the thalamus, which then projects back to the cortex. It is important to recognize that the basal ganglia plays an important role in a number of functions. For example, medications that are used to treat Parkinson’s can sometimes lead to the presentation of impulse control disorders, a result of dopaminergic changes in the limbic loop through the basal ganglia.

Key Takeaways
- The subcortical basal ganglia nuclei receive information from the cortex and send output to the thalamus
- Motor control through the basal ganglia occurs through both the direct and indirect pathways
- Disinhibition is when an inhibitory region is itself inhibited
- The basal ganglia are best known for their role in motor control but are also critical for emotion and behavioral inhibition
Test Yourself!
Attributions
Portions of this chapter were remixed and revised from the following sources:
- Foundations of Neuroscience by Casey Henley. The original work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License
- Open Neuroscience Initiative by Austin Lim. The original work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Media Attributions
- Basal Ganglia © Casey Henley adapted by Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Basal Ganglia Input © Casey Henley adapted by Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Basal Ganglia Input Text © Casey Henley adapted by Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Basal Ganglia Output © Casey Henley adapted by Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Basal Ganglia Output Text © Casey Henley adapted by Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Basal Ganglia Direct Pathway © Casey Henley adapted by Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Basal Ganglia Direct Pathway Text © Casey Henley adapted by Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Direct Pathway Activation © Casey Henley adapted by Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Direct Pathway Activation Text © Casey Henley adapted by Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Basal Ganglia Indirect Pathway © Casey Henley adapted by Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Basal Ganglia Indirect Pathway Text © Casey Henley adapted by Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Indirect Pathway Activation © Casey Henley adapted by Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- IndirectPathwayActivationText_lecture © Casey Henley adapted by Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Indirect Pathway Inhibition © Casey Henley adapted by Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Indirect Pathway Inhibition Text © Casey Henley adapted by Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Basal ganglia circuit model © Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Basal Ganglia Loops © Casey Henley adapted by Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license

