1.24: Surgery for Intrathoracic (Retrosternal) Goiter
- Page ID
- 17644
OPEN ACCESS ATLAS OF OTOLARYNGOLOGY, HEAD & NECK OPERATIVE SURGERY
SURGERY FOR INTRATHORACIC (RETROSTERNAL) GOITRES
Ricard Simo, Iain Nixon, Enyunnaya Ofo
Retrosternal, substernal and intrathoracic goitre is a subgroup of multinodular goiter (MNG). The most commonly recognised and appropriate term is intrathoracic goitre (IG) and for the purpose of this chapter this is the term that is used.
IG presents specific challenges in relation to preoperative evaluation and surgical management. Classic indications for surgery include pressure and cosmetic effects, and higher-than-expected rates of incidental malignancy in a MNG with retrosternal and intrathoracic extension1.
Multinodular goiter (MNG)(Figure 1)

Figure 1: Example of MNG
MNG refers to an enlarged thyroid gland with multiple nodules. Challenges specific to MNG include evaluating the patient, determining the risk of malignancy within the multiple nodules, selecting patients who require surgery, and planning an appropriate surgical approach to address the disease without undue risk of complications. MNG is generally presumed to be a response to oversecretion of Thyroid Stimulating Hormone (TSH). The thyroid hyperplasia is probably due to reduced production of thyroid hormones relative to the metabolic demand of the body. This can be due to a congenital or an acquired defect.
Morphological and molecular studies suggest a degree of polyclonal etiology. MNGs are sometimes familial and one study suggests linkage to a DNA mother as chromosome 14q2.
Not all MNGs require surgery. Palpable nodules occur in 4-7% of adults3. With the advent of high-resolution ultrasound (USS), nodules and nodular thyroids are detected in 50-70% of adults4. In noniodine deficient patients, ultrasound can detect thyroid nodules in >20% of people, and multiple nodules in 9%. Rates are higher in females and in older patients2,4. This suggests that an increasing number of patients with MNG will be encountered in an ageing population.
Intrathoracic Goiter (IG)
Concept and Classification
Haller first provided an anatomical description of the IG in 1794; since then it has been given several names and descriptions e.g. retrosternal, substernal, retroclavicular and intrathoracic goiter. Numerous classifications have been used (Table 1). Authors have compared these definitions in an attempt to define its utility and allow for sensible comparisons5. Huins et al indicated a need to apply 3 grades depending on the relationship of the IG to the aortic arch and the right auricle. More recently Rios et al critically analyzed all classifications to determine the most useful definition of IG for predicting intra-operative as well as postoperative complications. They found that most definitions can be ignored as they are not clinically relevant and concluded that Katlic’s definition6 was the most useful for predicting the need for a possible sternotomy to remove an IG7.

Table 1: Classifications of IG after Rios et al 2
Risk of malignancy
For a thyroid gland to reach sufficient size to pass into the mediastinum, the pathological processes involved must have been present for many years. It is therefore unsurprising that the vast majority of surgical specimens demonstrate benign pathology. The rate of malignancy in reported surgical series is low (6-21%)8, 9, 10, 11 . However, it is important to consider malignancy as it may significantly alter management.
Clinical presentation
It is not uncommon for IG to manifest in the elderly and many patients are relatively asymptomatic. The thoracic inlet, bound by the clavicles, 1st rib, sternum and vertebrae, contains many vital structures. In addition to the prevertebral muscles, the trachea, esophagus, carotid and jugular vessels all pass through this region. As the thyroid gland enlarges, an increasing percentage of the cross-sectional area of the inlet is occupied by the goiter, leaving less room for other structures. Pressure symptoms tend to develop in low-pressure areas in the first instance, and a feeling of difficulty swallowing is a common initial symptom. As the compression increases, along with increasing dysphagia, pressure on the trachea can lead to deformity of the tracheal rings and airway compression. In extreme cases, pressure on the venous structures of the neck can cause superior vena cava syndrome, although this is rare (5%)8. It manifests with distension of the veins in the neck, edema of the face and arms, shortness of breath and dysphagia. Elderly patients often have unrelated comorbidities and require a thorough preoperative evaluation and careful surgical planning and technique.
Airway and tracheomalacia
Airway management may not be straightforward, although truly difficult intubations are uncommon9. Cooperation between surgeon and anesthetist is crucial to avoiding problems at this critical stage of the procedure. The majority are amenable to endotracheal intubation, as the endotracheal tube stents the trachea open.
Some patients have symptoms that vary with head position. With the neck fully extended, the goitre is pulled upwards towards the thoracic inlet, and this might compromise the airway. In such cases an awake fiberoptic intubation may be required to allow the neck to be flexed during intubation.
Tracheomalacia is caused by longstanding pressure on the trachea and becomes evident only on completion of surgery. It manifests as stridor following extubation. It is uncommon, and postoperative tracheostomy is rarely required (2%); tracheostomy is more commonly required following traumatic intubation causing edema rather than for tracheomalacia9.
Indications for surgery
- Symptomatic (compression)
- Dysphagia
- Airway compression
- Superior vena cava syndrome
- Incidental: Detected with chest X-ray, USS, CT, MRI or PET
- Malignancy (suspected or confirmed)
Appropriate management should take into consideration the size of the goiter, the degree of aerodigestive tract compression, concerns about malignancy, and comorbidities.
With compressive symptoms, surgery provides the only means of relief and provides tissue for histological analysis. For the few patients with malignancy, resection is the mainstay of therapy, and permits adjuvant radioiodine treatment to be used when indicated.
Indications for surgery in patients in whom IG is incidentally found are unclear. As imaging becomes more widely used, an increasing number (up to 40%) of IGs are detected incidentally during workup of other diseases8,10. Some authors consider the mere presence of an IG as an indication for surgery9 whereas others question the need for surgery in all cases especially if malignancy is not suspected11. Decision-making regarding surgery in such patients should be individualised. For example, a patient with an asymptomatic IG detected on imaging to stage an incurable aggressive malignancy clearly is not a candidate for surgery. In contrast, an otherwise well patient with asymptomatic tracheal compression and an excellent life expectancy will be a good surgical candidate. Surgery in this clinical setting prevents progression of airway symptoms.
The difficult patient is one with little/no comorbidities and asymptomatic disease, with early tracheal compression. Such patients should be made aware of the risks and benefits of both a conservative and a surgical approach. Interval imaging often provides critical information about the trajectory of the disease which assists with decision making in borderline cases.
Preoperative evaluation
Once it has been decided that surgery is indicated, then preoperative evaluation focuses on the following:
Comorbidities: Because many patients are elderly, those requiring an extracervical approach (sternotomy / thoracotomy) need to be carefully assessed as to their general fitness for surgery. Electrocardiography, echocardiography and assessment of lung function should be considered in those requiring sternotomy, and in all other patients with significant cardiorespiratory disease as part of assessment prior to anesthesia.
Assessment of thyroid tumor: Patients should have a full head and neck examination with particular focus on the presence of cervical lymphadenopathy. Ultrasound is used to assess the central and lateral neck.
Fine Needle Aspiration Cytology (FNAC): Even though the incidence of malignancy with retrosternal MNG is low, (ultrasound guided) FNAC assessment may identify malignancy preoperatively and allow for better counselling and preoperative planning of the extent of surgery.
Thyroid and parathyroid function: Thyroid function tests and serum calcium levels are done. Hyperthyroid patients with are medically managed to achieve euthyroidism prior to surgery to prevent a life threatening thyrotoxic crisis during or after surgery. This usually involves thionamide antithyroid drugs, or potassium iodide (40 mg three times daily for 10 days) +/- beta-blockade (e.g. propranolol 40-80 mg three times per day).
Vocal cord function: Assessing vocal cord function by indirect or flexible laryngoscopy in the awake patient is essential, not only for medicolegal reasons, but also to determine the function of the contralateral RLN to pre-empt possible airway compromise postoperatively.
Imaging the trachea: In patients with stridor due to tracheal compression, imaging is required to determine the site and length of the tracheal narrowing both for the anesthetist and the surgeon. Even though CT or MRI is preferred, an AP Xray may give a good indication (Figure 3).
Imaging the mediastinum (Figures 4, 5): The relationship of a goiter to the trachea, esophagus and great vessels is readily appreciated on imaging, and guides the surgical approach (cervical +/- sternotomy) which may require the input of other surgical teams, as well as provides invaluable information to the anesthetist about the presence of laryngotracheal compression and likely problems with endotracheal intubation.
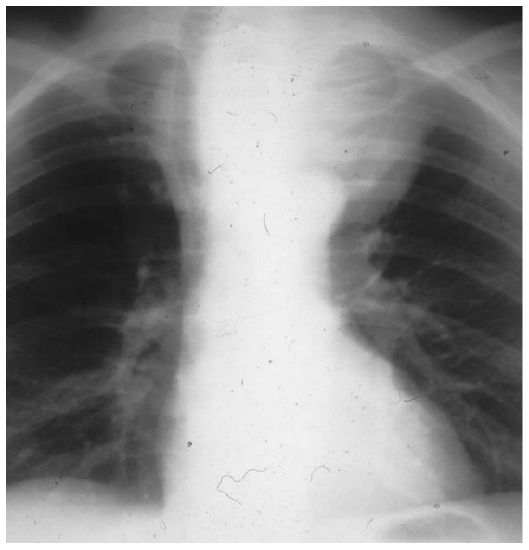
Figure 3: X-ray shows tracheal compression and displacement to the right
Patients with (suspected) retrosternal thyroid should have cross-sectional imaging (CT or MRI) with intravenous contrast to define its size, position and anatomical relationships to mediastinal structures. Imaging helps to demonstrate the tissue planes surrounding the thyroid mass. Any evidence of extrathyroidal extension should be considered as evidence of malignancy and the surgical approach tailored accordingly8.
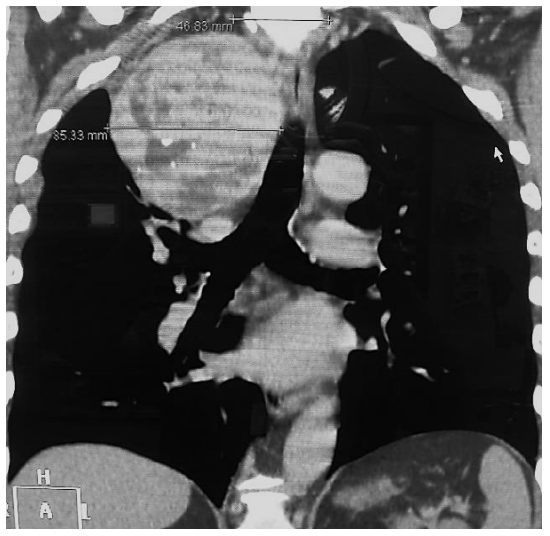
Figure 4: CT of intrathoracic goiter MNG with displacement of trachea to left side

Figure 5: MRI of malignant tumor with poorly defined tissue planes and invasion of internal jugular and brachiocephalic veins
Coagulation status: Exclude clotting disorders to reduce bleeding, discontinue anticoagulants e.g. warfarin or clopidogrel and substitute with heparin if required
Multidisciplinary team meeting: Once a patient satisfies the indications for IG surgery the multidisciplinary team must set aside time to ensure that the patient and conditions are favorable to proceed and to plan to minimize complications. IG can be associated with significant laryngotracheal compression resulting in difficult orotracheal intubation. Prior to surgery, the surgeon and anesthetist must discuss the airway plan and jointly review the imaging.
Consent
Informed consent is essential. Complication rates from IG surgery are low in experienced hands, but when they do occur, they may be associated with significant morbidity and/or mortality. Patients need to be aware of these risks, especially in the context of an asymptomatic patient with benign disease who may elect to delay surgery and be managed conservatively. However in the context of retrosternal goiter, patients should also be aware that with continued growth, delaying an operation may make future surgery technically more challenging, thus potentially increasing the risk of complications.
Prior to discussing specific risks of surgery, patients are informed of general anesthetic (GA) risks. When the goitre is associated with significant tracheal compression, orotracheal tube intubation may be difficult, and patients should be enlightened that initial airway management may be challenging, though with appropriate preoperative planning and medical/surgical personnel, this should not be insurmountable.
IG surgery is associated with a number of potential life-threatening complications although in specialized centers complication rates are usually low (<5% for bleeding, infection, permanent recurrent laryngeal nerve (RLN) and external branch of superior laryngeal nerve (EBSLN) paralysis and hypoparathyroidism)12,13. However it is important that the operating thyroid surgeon regularly audits his/her own complication rates, and informs patients of their own outcomes.
The functional implications of RLN palsy include altered voice, aspiration, or airway obstruction if bilateral. EBSLN palsy may also cause vocal fatigue and impairment of voice pitch and projection. Unrecognized hypoparathyroidism and consequent hypocalcemia may be life threatening. Permanent hypoparathyroidism requires lifelong calcium +/- vitamin D supplementation. In addition, patients need to be made aware of the need for lifelong thyroxine with total thyroidectomy, and unless undergoing a robotic or other minimally invasive approach, that they will have a neck scar that in some ethnic groups, such as Asians or Afro-Caribbeans may become hypertrophic or form keloid. Regardless of surgical approach, there will also be numbness above or below the incision and that may be permanent.
IT surgery may require median sternotomy to remove the superior mediastinal component. Thoracic surgeons usually perform the sternotomy. Patients need to be aware that although uncommon (<5% of patients) complications from median sternotomy may include trauma to mediastinal structures, pneumothorax, pneumomediastinum, mediastinitis, sternal dehiscence, and osteomyelitis.
Anesthesia
Patient position: We previously alluded to possibly worsening airway obstruction by extending the neck; this situation may require blind fiberoptic intubation in an awake patient.
Intubation technique: Extrinsic tracheal compression is generally ‘soft ‘in nature and can easily be overcome by gentle insertion of an endotracheal tube, which may need to be one size smaller than what is usually used for a patient. Occasionally a stiff introducer may be required to forcefully advance the tube past a narrowed tracheal segment. To avoid the dreaded emergency scenario of ‘can’t intubate, can’t ventilate’ at induction of anesthesia in a paralyzed patient, the anesthetist may choose to perform an awake fiberoptic oral/nasal tracheal intubation with the aid of topical local anesthesia.
Choice of endotracheal tube: The anesthetist must be aware of the presence, degree and level of compression of the trachea to select and appropriate diameter, and length of tube. A double lumen endotracheal tube may be required to permit selective pulmonary ventilation. Cases where extensive mediastinal dissection is anticipated require an experienced anesthetist with appropriate head and neck or thoracic anesthetic expertise, as these tubes can be challenging to place in patients with a difficult airway.
Neuromonitoring: Neuromonitoring of the recurrent laryngeal nerve reduces palsy rates with difficult thyroidectomy, such as in retrosternal goitre surgery14. Where nerve monitoring is to be employed, longacting muscle relaxants must be avoided so as not to interfere with neural monitoring; short acting agents can be used for induction of anesthesia.
Extubation: At the end of surgery, tracheal compression from a longstanding goiter may cause a degree of tracheamalacia; in our experience extubation is almost always possible. In the highly unlikely event that the patient experiences airway obstruction on extubation due to tracheomalacia, the patient should be reintubated for a minimum of 72 hours until a leak is observed around the endotracheal tube and a tracheostomy performed if necessary20.
Postoperative monitoring: Patients with significant cardiorespiratory disease requiring median sternotomy are at higher risk of postoperative complications and require close monitoring after surgery in a high dependency or intensive care setting.
Choice of Surgical Approach
Surgery for IG poses significant intra- and postoperative challenges and should ideally be done by experienced surgeons who are part of a multidisciplinary team and have the knowledge and ability to deal with intra- and postoperative complications of the surgery.
Total vs hemithyroidectomy?
Total thyroidectomy is indicated for bilateral thyroid gland enlargement. However, in patients with unilateral enlargement or in whom there is a significant risk to the RLN or parathyroid function, thyroid lobectomy is a perfectively acceptable option as most goiters will be benign9. Total thyroidectomy is also contraindicated in settings where thyroid and/or calcium replacement therapy and monitoring is not possible as is the case in many developing countries.
Cervical vs. Extracervical approach?
- Cervical: Suitable for 95% of IGs.
- Extracervical: The need for sternotomy increases greatly if a significant proportion of the gland is located in the mediastinum or the IG is in a retrotracheal or retro-esophageal position, or if the intrathoracic component is significantly larger than the cervical one. The main indications for an extracervical approach therefore are giant intrathoracic extension, recurrent goiters, presence of malignancy with extrathyroidal extension, extension posterior to trachea and esophagus, extension between trachea and esophagus, Isolated mediastinal goiters and intrathoracic goiters with diameter greater than the diameter of the thoracic inlet.
Surgical Technique
Management of RLN: The RLN should always be identified. With large goiters, localization of the nerve can be challenging due to distortion of the anatomy. It is not uncommon for the nerve to be riding over a hyperplastic nodule, making it particularly vulnerable. With this in mind, surgeons must be familiar with localizing the RLN either at the cricothyroid joint (Figure 6) or laterally at the level of the inferior thyroid artery (ITA).

Figure 6: Right recurrent laryngeal nerve (arrow) identified at cricothyroid joint and followed distally using “toboggan technique” as described by Charles Proye
Neuromonitoring: Neuromonitoring for neoplastic MNG is a valuable technique to identify and safely preserve the RLN.
Parathyroid glands: It is imperative to ensure that every effort is made to identify and preserve at least the superior glands which have a more constant anatomical position in close relationship with the ITA. With surgery for large MNGs the parathyroids may be displaced due to aberrant growth of the thyroid gland.
Berry’s ligament: Dissection of Berry’s ligament is one of the most delicate steps of thyroid surgery due to its vascularity and close relationship to the RLN. It is imperative that minimal traction be applied when holding the thyroid lobe to avoid traction injury to the nerve. The ligament is dissected from the nerve with fine instruments, usually with judicious use of bipolar diathermy and fine scalpel dissection.
The following surgical approaches will now be described:
- Cervical
- Extracervical
- Combined cervical & midline sternotomy
- Combined cervical & lateral thoracotomy
1. Cervical Approach
Surgery for MNG can be challenging due to distortion of anatomy, difficult exposure, and involvement of vital structures.
- Incisions: In patients with MNG with retrosternal extension, make a generous extended Kocher incision in the lower neck; the Kocher incision, placed midway between the cricoid and sternal notch, is extended in a skin crease up to the anterior border of the trapezius muscle (Figure 7).

Figure 7: Extended Kocher’s incision (yellow), Modified Kocher’s incision (red), and vertical incision for sternotomy (green)
If, however, a lateral neck dissection is done then a modified extended Kocher incision is preferred (Figure 7); this a similar incision as described above, other than that the incision is placed centered over the cricoid prominence to permit exposure of the neck up to the mandible. If a midline sternotomy is required, then a minor vertical incision is added (Figure 7)
- Elevate subplatysmal flaps laterally up to the trapezius muscle and inferiorly to the clavicle and the sternal notch to allow access to the goiter at the thoracic inlet
- Thyroid isthmus: Identify and skeletonize and divide the isthmus. If a lobectomy is to be done, the isthmus is divided early as this facilitates the cervical dissection. With very big bilateral goiters, this can be done early for the same reason, so the procedure becomes essentially two lobectomies. With total thyroidectomy the dissection should start with the smaller lobe as facilitates subsequent dissection of the larger lobe. In selected cases, following lobectomy on the side of the smaller lobe, divide the isthmus to allow better mobilization of the dominant lobe, reducing cervical pressure and helping to locate the RLNs and the parathyroid glands more easily
- Strap muscles: In large MNGs, the strap muscles, in particular the sternothyroid, may be divided to achieve better control of regional veins, improve exposure to the lateral aspect of the goiter and superior vascular pedicle, and to provide better access to mobilize the goiter and to visualize the anatomical structures that must be preserved
- Middle thyroid vein: Identify, dissect, ligate and divide the middle thyroid vein. Avoid rough manipulation of the gland as this may avulse the vein from the internal jugular vein
- Superior thyroid pole: Dissect the thyroid lobe from the prethyroid strap muscles and divide the sternothyroid muscle for access to the upper pole. Identify the superior thyroid pole and ligate the individual superior thyroid vessels close to the gland to avoid injury to the external branch of the superior laryngeal nerve. Dissect the superior pole from its attachments to the cricothyroid muscle
- RLN: Once the upper pole is dissected and mobilized, the RLN is identified at the cricothyroid junction and dissected in a caudal direction tunneling the tissue surrounding the nerve with a fine-tipped mosquito dissector (Figure 6). The RLN is dissected inferolaterally as much as the approach allows it under the common carotid artery and brachiochepalic artery and gently controlled with a rubber vessel sling. However, depending on the shape and size of the goiter, the nerve may also be identified in the lateral or inferior position in Beahr’s triangle and followed cranially to the cricothyroid joint and caudally to the mediastinum (Figure 8).

Figure 8: Right RLN identified at Beahr’s triangle and followed cranially to cricothyroid joint
- Thyroid lobectomy: Free the thyroid lobe from its cervical attachments (esophagus & trachea) as much as possible up to the thoracic inlet so that it is freed from its superior mediastinal attachments
- Parathyroid glands: The parathyroid glands are closely related to the thyroid and may inadvertently be removed even by experienced surgeons. It is more likely to occur with retrosternal goiter surgery as the altered anatomy makes it difficult to locate the parathyroid glands. Every attempt should be made to identify the parathyroid glands in their usual positions close to the inferior thyroid artery and RLN, while preserving their blood supply. The excised thyroid gland should also be carefully inspected for the presence of a parathyroid glands that should then be re-implanted into the sternomastoid muscle
2. Extracervical Approaches
- Combined Cervical & Midline sternotomy
- Combined Cervical & Lateral Thoracotomy
Extracervical approaches require an understanding and experience of surgical techniques to access the mediastinum and the pleural spaces as well as having a dedicated and expert multidisciplinary team including (cardio)thoracic surgeons.
Combined Cervical & Midline Sternotomy
Indications for sternotomy are based on the anatomical relationship of the goitre to mediastinal structures. The indications have already been described above. It comprises 3 main stages:
- Cervical stage
- Sternotomy and mediastinal stage
- Thoracic inlet stage
- Cervical Stage
- Make a standard Kocher (transverse) skin incision (Figure 7)
- Elevate subplatysmal flaps
- Proceed with the dissection as previously described under “Cervical approach"
- Sternotomy and Mediastinal Stage
- Completely expose the chest and prepare the skin from neck-to-umbilicus and axilla-to-axilla
- Make a midline incision from the cervical wound to the xiphisternum in a T-fashion (Figure 7)
- Incise the subcutaneous fat down to the sternal periosteum with cautery or a scalpel
- Identify the midline superiorly at the sternal notch, inferiorly at the xiphisternum and midway along the sternum by digitally palpating the intercostal spaces
- Make a linear incision in the sternal periosteum from top-to-bottom with electrocautery keeping precisely in the midline in preparation for the saw (Figure 9)
- Divide the xiphisternum with curved Mayo scissors and the suprasternal ligament with electrocautery (Figure 10)
- Bluntly dissect with a finger and sweep retrosternally at the top and bottom ends of the sternum to expose and prepare a space for the path of the saw
- Instruct the anesthetist to stop ventilating the patient while the sternum is split along its length with the saw
- Resume ventilation and control bleeding from the periosteal edges with electrocautery
- Insert a Holmes-Sellors retractor to expose the mediastinum

Figure 9: T-shaped cervico-thoracic incision
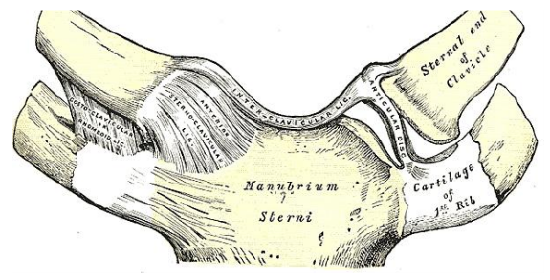
Figure 10: Suprasternal / interclavicular ligament

Figure 11: Holmes-Sellors retractor
- Inspect the mediastinum to clarify the location and extent of the goiter
- Identify the brachiocephalic or innominate vessels and control them with soft rubber vascular slings if necessary
- Take care to minimize unnecessary pleural or pericardial breaches especially if malignancy is suspected. In such cases it is advisable to start the dissection as caudally as possible to clear out mediastinal fat containing lymph nodes
- Once the goiter is identified and mediastinal vessels controlled, commence the dissection in an extracapsular plane anteriorly-to-inferiorly, ligating any extracapsular vessels that are encountered (Figure 12)
- Proceed posteriorly and laterally with the dissection
- Deliver the goiter in an upward direction until the thoracic inlet is reached
- Inspect the cavity for bleeding, achieve hemostasis, and ensure that no mediastinal structures have inadvertently been injured

Figure 12: Mediastinal stage with mediastinal dissection of the IG
3. Thoracic inlet stage
- With the thyroid gland mobilized superiorly and inferiorly, commence the dissection to mobilize the gland at the thoracic inlet
- This is the narrowest part of the dissection and leaving it until last allows for easier mobilization of the gland. It also allows better visualization of the RLN which should be carefully dissected from the remaining gland
- Once the thyroidectomy is completed, the wound is washed with warm saline solution. Hemostasis is achieved and a Valsalva manoeuvre is used to ensure that there are no bleeding points
- One or two 28 FG Rocket ® mediastinal drains are inserted up to the level of the thoracic inlet, so the neck also drains adequately, and set at 2-3 kilopascal negative pressure
- Close the wound in layers with absorbable sutures for the platysma and strap muscles
- Close the sternum with several standard titanium wires
- Close the sternal skin incision with subcuticular non-absorbable sutures and with metallic staples for the cervical incision
Combined Cervical & Lateral Thoracotomy
This approach has 2 main stages:
i. Cervical Stage
ii. Thoracotomy, and posterior mediastinal dissection stage It is indicated when a goiter extends into the posterior mediastinum and reaches the posterior pleura and a midline sternotomy would not provide enough space to dissect the goiter from such a posterior location (Figure 13).
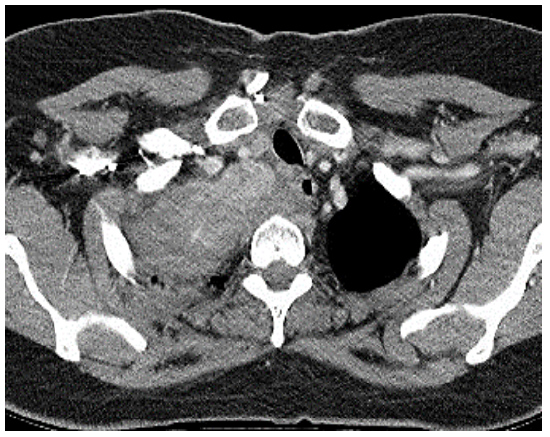
Figure 13: IG with extension to posterior pleura
i. Cervical Stage
- The cervical stage follows the same steps as previously described
- Free the thyroid from its cervical attachments (esophagus and trachea) into the thoracic inlet as much as is possible, and from its upper mediastinal attachments
- Amputate the cervical portion of the thyroid using e.g. a Harmonic Scalpel
- Pack 2 or 3 layers of Surgicel ® Fibrillar absorbable hemostat between the RLN and the intrathoracic portion of the goitre to protect the nerve during the final stages of the thoracotomy approach
ii. Thoracotomy & posterior mediastinal dissection stage
- Reposition the patient in a lateral position, but rolled back to simultaneously allow access to the anterior neck and the thoracotomy as needed
- Make a high posterolateral thoracotomy, usually on the right side as the aortic arch and its branches impede access on the left side
- Divide latissimus dorsi and preserve serratus anterior
- Enter the chest through the 4th intercostal space (Figure 14)

Figure 14: Lateral thoracotomy with exposure of posterior pleura and posterior aspect of IG
- It is generally necessary to separate the goiter from the superior vena cava anteriorly and from the trachea, taking care not to injure the phrenic nerve
- Take special care not to injure the right RLN as it recurs around the great vessels at the thoracic inlet. The layer of Surgicel ® fibrillar protects the nerve at this stage
- Divide Sibson’s fascia as doing so usually makes it possible to join the thoracic and cervical dissections
- Lower down, the innominate vein is often stretched across the goiter; when mobilizing it, take care not to tear the vein or to avulse the veins draining the goiter
- Mobilize the goiter; this is usually best achieved by gentle blunt dissection of the pseudocapsule to avoid tearing the goiter and its feeding veins
- Control bleeding from the vascular surface with packing during the dissection, or with bipolar cautery
- Once the excision is complete, care is taken to ensure hemostasis
- The wound is washed with warm normal saline solution
- A size 28 FG Rocket ® chest drain is inserted and set at 2-3 kilopascals of negative pressure
- The wound is then closed in layers in a standard fashion.
- In the postoperative period regular chest physiotherapy is given to prevent postoperative pneumonia.
Consequences & Complications
Postoperative complications are infrequent in experienced units but need to be recognized and managed timeously to minimize morbidity and mortality.
Post-extubation airway obstruction
With bilateral surgery, transient or permanent bilateral RLNs paralysis may cause life threatening airway obstruction. It may not always be immediately evident following extubation but should be suspected with stridor and respiratory compromise following total thyroidectomy, or with a unilateral procedure in a setting of preexisting contralateral RLN palsy. In cooperative patients, flexible laryngoscopy confirms the diagnosis. Management depends on the degree of respiratory compromise and the surgeon’s level of confidence that the RLN paralysis is transient or permanent. This is why neuromonitoring is useful, as a nerve for which an intact monitoring circuit had been confirmed with pre- and post-dissection vagal stimulation will invariably recover 15. A decision then has to be made to either to institute conservative measures (e.g. supplemental oxygen, adrenaline nebulisers) and to closely monitor the patient’s airway, or to reintubate the patient or to do a tracheostomy.
Bleeding & Hematoma
Bleeding may manifest as increased drain output (e.g. >100 mL in <1 hour) and/or an expanding neck hematoma. Both situations require immediate re-exploration of the neck to arrest bleeding to avoid both airway and cardiovascular compromise. Most hematomas occur in the 1 st 24 hours; bilateral surgery has a higher risk compared to thyroid lobectomy. Life-threatening hematomas are uncommon and occurs in <1% of cases 31. Preoperative evaluation should have identified patients at high risk of bleeding such as those on anticoagulants. An expanding neck hematoma reduces venous return and can quickly cause airway obstruction due to laryngeal edema. Management includes early recognition and immediate evacuation of the hematoma and arresting the source of the bleeding. Airway management may require a tracheostomy or cricothyroidotomy if endotracheal intubation is unsuccessful.
Hypocalcemia
The parathyroid glands may inadvertently be removed even by experienced surgeons. The excised thyroid gland should carefully be inspected for the presence of parathyroid glands that should then be reimplanted into the sternomastoid muscle. Hypoparathyroidism needs to be detected to prevent cardiovascular and neurological complications from hypocalcemia. Following total thyroidectomy, it is common practice to check the adjusted serum calcium levels 6 and 12 hours postoperatively. Levels <1.9 mmol/L should prompt intravenous calcium to be administered (usually 10mls of 10% calcium gluconate over 10-15 minutes) to prevent cardiovascular and neurological consequences of hypocalcaemia. When the surgeon strongly suspects that the parathyroids were compromised during surgery e.g. following central compartment neck dissection, one may elect to commence oral calcium supplements +/- vitamin D immediately postoperatively. Serum parathyroid hormone (PTH) levels can be checked to determine the need for long-term oral calcium supplementation. Patients sent home on oral calcium should be closely followed up to ensure that an appropriate early calcium weaning protocol is followed in patients (majority) that recover normal parathyroid function.
Seroma
Seromas may be reduced if neck drains are used 16, and not removed too early. Despite appropriate drain management, seromas however still occur. Seromas usually settle within 4-6 weeks. Active management by repeated aspiration is only required when for compressive symptoms or cosmetic concerns.
Infection
Because thyroidectomy is a “clean” operation, infection is uncommon and prophylactic antibiotics are not recommended 13. When it does occur, it usually settles with antibiotics that cover Staphylococcal and/ or Streptococcal bacteria. Very rarely is incision and drainage of an abscess required.
Vocal cord dysfunction
Voice change occurs commonly following thyroidectomy. It is not necessarily due to injury to the RLN but is often caused by laryngeal edema from the endotracheal tube, and postsurgical venous congestion of the larynx. Apart from altered voice that occurs with RLN injury, patients may experience swallowing difficulty, aspiration, and life-threatening stridor with bilateral RLN paralysis
Vocal cord paralysis due to RLN injury is a potentially serious complication. In cases of retrosternal goitre, the external branch of the superior laryngeal nerve (EBSLN) and RLN are at greater risk due to being displaced by the tumor; this makes anatomical recognition more difficult. Additionally the maneuvers required to deliver a large goiter render the RLN more susceptible to stretching or compression.
Always examine the vocal cords at follow-up not only to assess functional outcome of the surgery, but also because of implications of a paralyzed RLN for swallowing and future contralateral surgery.
When the RLN has obviously been transected intraoperatively, most agree that a tension-free repair of the RLN should be performed.
Airway and swallowing dysfunctions are managed in the perioperative period depending on the status of the contralateral RLN. However, when vocal cord paralysis occurs when the RLN was noted to be anatomically intact at completion of surgery (+/- normal function confirmed with neuromonitoring), then re-exploration is not indicated as the paralysis is invariably temporary. Dexamethasone may possibly reduce the rate of temporary RLN palsy 17.
With bilateral RLN injury, airway compromise is to be expected. Management is dictated by the intraoperative assessment of the RLNs. If the nerves were intact, then patients should be reintubated and managed in a critical care setting with intravenous steroids and a trial of extubation attempted after 48 hours.
Voice therapy by a speech and language therapist/pathologist is an essential aspect of management of RLN or EBSLN injury. Principles of voice therapy include ensuring understanding and knowledge of laryngeal function and rationale of therapy; voice care, managing glottal insufficiency; improving vocal fold adduction; reducing transglottic airflow and improving subglottic air pressure; preventing supraglottic hyperfunction; optimizing resonance and pitch; reducing aspiration; and improving airway clearance. This is best initiated as early as possible for best outcomes. In a small minority of patients with permanent unilateral vocal cord paralysis that do not respond to voice therapy, a variety of vocal cord medialization procedures can improve voice and swallowing.
Most patients in whom bilateral RLN injury is recognized intraoperatively require immediate tracheostomy to secure a safe airway. Further management depends on patient and tumor factors. Further interventions may not be indicated in patients with advanced disease or significant comorbidities. Laser cordotomy may allow decannulation of fit, motivated patients with reasonable pulmonary reserve.
Thyroid hormone replacement
Hemithyroidectomy is associated with a 12-35% risk of hypothyroidism 18, 19. Hence patients should have thyroid functions checked within 6-8 weeks. Risk factors for hypothyroidism include high-normal serum TSH levels, lower free thyroxine levels and Hashimoto’s thyroiditis 20, 21. Lifelong thyroid hormone replacement is required following total thyroidectomy, usually in the form of oral levothyroxine, with doses adjusted over a 2-8 weeks to achieve optimum therapeutic levels.
Tracheomalacia
Tracheomalacia refers to weakness of the tracheal wall following resection of a goiter. Although weakness of the cartilaginous trachea has been attributed to longstanding compression causing ischemic damage, the pathophysiology remains poorly understood.
Tracheomalacia causing airway obstruction is very rare. A soft, easily collapsible trachea following goiter resection should be noted on palpation, following which a tracheostomy should be performed to secure the airway. If tracheomalacia was suspected intraoperatively and the patient develops airway obstruction following extubation, it is important to exclude bilateral RLN paralysis. If bilateral RLN paralysis has been excluded, repeat endotracheal intubation is done with a trial of extubation after 48 hours. Waiting 48 hours allows paratracheal fibrosis to occur that reduces tracheal collapse from negative intratracheal pressures. However, one should have a low threshold for tracheostomy for medium to long-term airway management.
Relevant Open Access chapters
Thyroidectomy under local and regional (cervical plexus block) anesthesia
References
- Nixon IJ, Simo R. The neoplastic goitre. Curr Opinion Otolaryngol Head Neck Surg 2013;21(2):143-9
- Association BT. Guidelines for the Management of Thyroid Cancer (2nd Edition). In: Physicians RCo, editor. 2nd ed. Great Britain: The Lavenham Press, Suffolk; 2007. p92
- Ezzat S, Sarti DA, Cain DR, Braun-stein GD. Thyroid incidentalomas. Prevalence by palpation and ultrasonography. Arch Intern Med 1994;154 (16):1838-40
- Brix TH, Hegedus L. Genetic and environmental factors in the aetiology of simple goitre. Ann Med 2000;32 (3):153-6
- Rios A, Rodriguez JM, Balsalobre MD, Tebar FJ, Parrilla P. The value of various definitions of intrathoracic goiter for predicting intra-operative and postoperative complications. Surgery 2010; 147(2):233-8
- Rugiu MG, Piemonte M. Surgical approach to retrosternal goitre: do we still need sternotomy? Acta Otorhinolaryngologica Italica: Organo ufficiale della Societa Italiana di Otorinolaringologia e Chirurgia Cervico-faciale 2009;29(6):331-8
- Huins CT, Georgalas C, Mehrzad H, Tolley NS. A new classification system for retrosternal goitre based on a systematic review of its complications and management. Int J Surg 2008;6 (1):71-6
- Katlic MR, Grillo HC, Wang CA. Substernal goiter. Analysis of 80 patients from Massachusetts General Hospital. Am J Surg 1985;149(2):283-7
- Cohen JP. Substernal goiters and sternotomy. Laryngoscope 2009;119(4): 683-8
- Coskun A, Yildirim M, Erkan N. Substernal goiter: when is a sternotomy required? Int Surg 2014;99(4):419-25
- Vasica G, O'Neill CJ, Sidhu SB, Sywak MS, Reeve TS, Delbridge LW. Reoperative surgery for bilateral multinodular goitre in the era of total thyroidectomy. Br J Surg 2012;99(5): 688-92
- White ML, Doherty GM, Gauger PG. Evidence-based surgical management of substernal goiter. World J Surg 2008;32 (7):1285-300
- McKenzie GA, Rook W. Is it possible to predict the need for sternotomy in patients undergoing thyroidectomy with retro sternal extension? Interactive Cardiovascular and Thoracic Surgery 2014;19(1): 139-43
- Goncalves FJ, Kowalski LP. Surgical complications after thyroid surgery performed in a cancer hospital. Otolaryngol Head Neck Surg 2005;132(3): 490-4
- Randolph GW, Kobler JB, Wilkins J. Recurrent laryngeal nerve identification and assessment during thyroid surgery: laryngeal palpation. World J Surg 2004;28(8):755-60
- Darr EA, Randolph GW. Management of laryngeal nerves and parathyroid glands at thyroidectomy. Oral Oncol 2013; 49(7): 665-70
- Hisham AN, Lukman MR. Recurrent laryngeal nerve in thyroid surgery: a critical appraisal. ANZ J Surg 2002;72 (12):887-9
- Atkinson HS, S. Nerve monitoring in thyroid and salivary gland surgery. J ENT Masterclass 2013;6(1):99-103
- Sancho JJ, Kraimps JL, Sanchez-Blanco JM, et al. Increased mortality and morbidity associated with thyroidectomy for intrathoracic goiters reaching the carina tracheae. Arch Surg 2006;141(1):82-5
- Randolph GW, Dralle H, Abdullah H, et al. Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: international stan dards guideline statement. Laryngoscope 2011;121 Suppl 1:S1-16
- Lorente-Poch L, Sancho JJ, Ruiz S, Sitges-Serra A. Importance of in situ preservation of parathyroid glands during total thyroidectomy. Br J Surg 2015;102 (4):359-67
Authors
Ricard Simo FRCS (ORL-HNS)
Consultant Otorhinolaryngologist, Head & Neck Surgeon
Guy’s and St Thomas’ Hospital NHS Foundation
Trust and Honorary Senior Lecturer
Guy’s, King’s and St Thomas’ Medical School
London, United Kingdom
ricard.simo@gstt.nhs.uk
Iain J. Nixon, MBChB, FRCS (ORLHNS), PhD
Consultant Otorhinolaryngologist, Head & Neck Surgeon
Edinburgh Royal Infirmary, United Kingdom
iainjnixon@gmail.com
Enyunnaya Ofo FRCS (ORL-HNS), PhD
Consultant Otorhinolaryngologist, Head & Neck Surgeon
St George’s University Hospital
London, United Kingdom
eofo@hotmail.com
Editor
Johan Fagan MBChB, FCS(ORL), MMed
Professor and Chairman
Division of Otolaryngology
University of Cape Town
Cape Town, South Africa
johannes.fagan@uct.ac.za


