6.3: Lipid Metabolism Pathways
- Page ID
- 40961
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Five lipid metabolic pathways/processes will be covered in the following subsections.
Lipolysis (Triglyceride Breakdown)
Lipolysis is the cleavage of triglycerides to glycerol and fatty acids, as shown below.

There are two primary lipolysis enzymes:
- Lipoprotein lipase (LPL)
- Hormone-sensitive lipase (HSL)
Despite performing the same function, at the adipose level, the enzymes are primarily active for seemingly opposite reasons. In the fed state, LPL on the endothelium of blood vessels cleaves lipoprotein triglycerides into fatty acids so that they can be taken up into adipocytes, for storage as triglycerides, or myocytes where they are primarily used for energy production. This action of LPL on lipoproteins is shown in the two figures below.
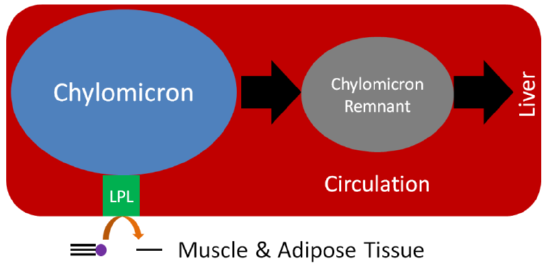
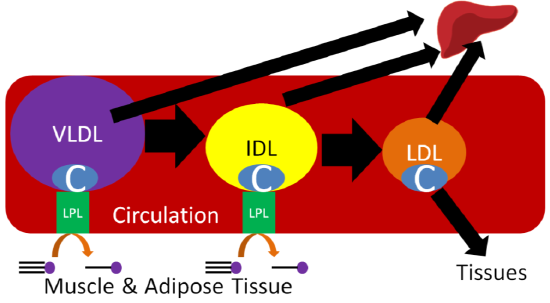
Query \(\PageIndex{1}\)
HSL is an important enzyme in adipose tissue, which is a major storage site of triglycerides in the body. HSL activity is increased by glucagon and epinephrine ("fight or flight" hormone), and decreased by insulin. Thus, in hypoglycemia (such as during a fast) or a "fight or flight" response, triglycerides in the adipose are cleaved, releasing fatty acids into circulation that then bind with the transport protein albumin. Thus, HSL is important for mobilizing fatty acids so they can be used to produce energy. The figure below shows how fatty acids can be taken up and used by tissues such as the muscle for energy production1.
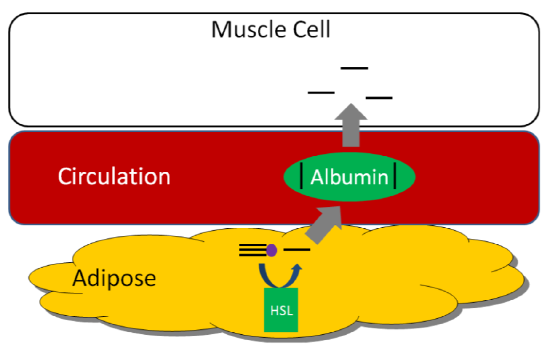
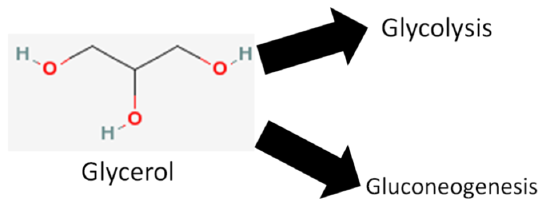
We are not going to focus on glycerol, but it does have two metabolic fates.
- It can be broken down in glycolysis
- It can be used to synthesize glucose (gluconeogenesis)
Query \(\PageIndex{2}\)
Fatty Acid Oxidation (Beta-oxidation)
To generate energy from fatty acids, they must be oxidized. This process occurs in the mitochondria, but long chain fatty acids cannot diffuse across the mitochondrial membrane (similar to absorption into the enterocyte). Carnitine, an amino acid-derived compound, helps shuttle long-chain fatty acids into the mitochondria. The structure of carnitine is shown below.

Fatty Acid Shuttling
As shown below, there are two enzymes involved in this process: carnitine palmitoyltransferase I (CPTI) and carnitine palmitoyltransferase II (CPTII). CPTI is located on the outer mitochondrial membrane, CPTII is located on the inner mitochondrial membrane. The fatty acid is first activated by adding \(\ce{CoA}\) (forming acyl-\(\ce{CoA}\)), then CPTI adds carnitine. Acyl-Carnitine is then transported into the mitochondrial matrix with the assistance of the enzyme translocase. In the matrix, CPTII removes carnitine from the activated fatty acid (acyl-\(\ce{CoA}\)). Carnitine is recycled back into the cytosol to be used again, as shown in the figure below. Even though carnitine is important for this action, taking supplemental carnitine will not increase fatty acid oxidation. This is due to the fact that the amount of carnitine available is not limiting fatty acid oxidation.

Query \(\PageIndex{3}\)
Fatty Acid Activation
As shown below, the first step of fatty acid oxidation is activation. A \(\ce{CoA}\) molecule is added to the fatty acid to produce acyl-\(\ce{CoA}\), converting ATP to AMP (adenosine monophosphate). Thus, activation uses the equivalent of 2 ATP molecules (since it typically cleaved to ADP)5.
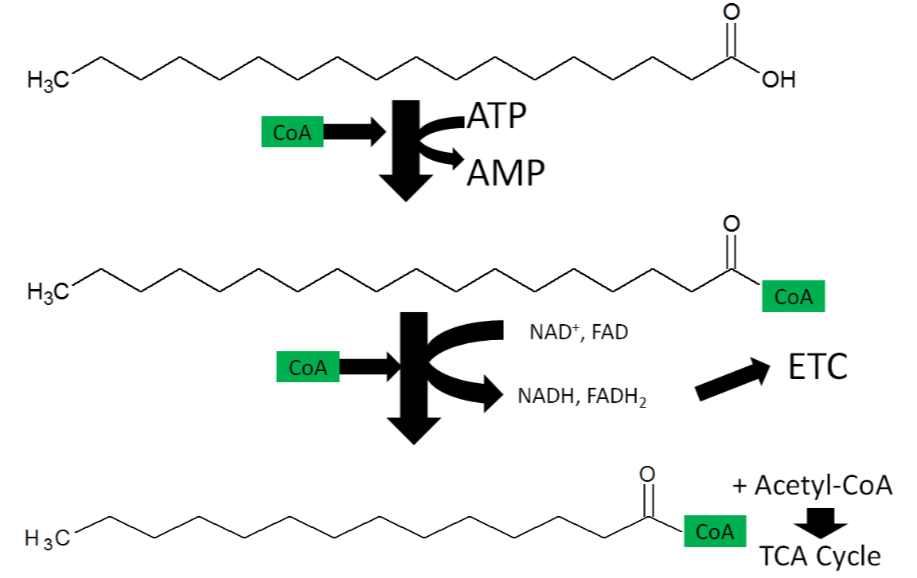
Fatty Acid Oxidation
Fatty acid oxidation is also referred to as beta-oxidation because 2 carbon units are cleaved off at the beta-carbon position (2nd carbon from the acid end) of an activated fatty acid. The cleaved 2 carbon unit forms acetyl-\(\ce{CoA}\) and produces an activated fatty acid (acyl-\(\ce{CoA}\)) with 2 fewer carbons, acetyl-\(\ce{CoA}\), \(\ce{NADH}\), and \(\ce{FADH2}\).
To completely oxidize the 18-carbon fatty acid above, 8 cycles of beta-oxidation have to occur. This might seem like one too few cycles (18 divided by 2 is nine), but the last cycle will split the 4 carbon fatty acid into 2 acetyl-\(\ce{CoA}\)s, meaning that it only takes 8 cycles to completely cleave the fatty acid. Overall beta oxidation of an 18 carbon fatty acids will produce:
9 acetyl-\(\ce{CoA}\)s
8 \(\ce{NADH}\)
8 \(\ce{FADH2}\)
Those 9 acetyl-\(\ce{CoA}\)s can continue into the citric acid cycle, where they can produce:
9 GTP
9 \(\ce{FADH2}\)
27 \(\ce{NADH}\)
The products of the complete oxidation of a fatty acid are shown below.
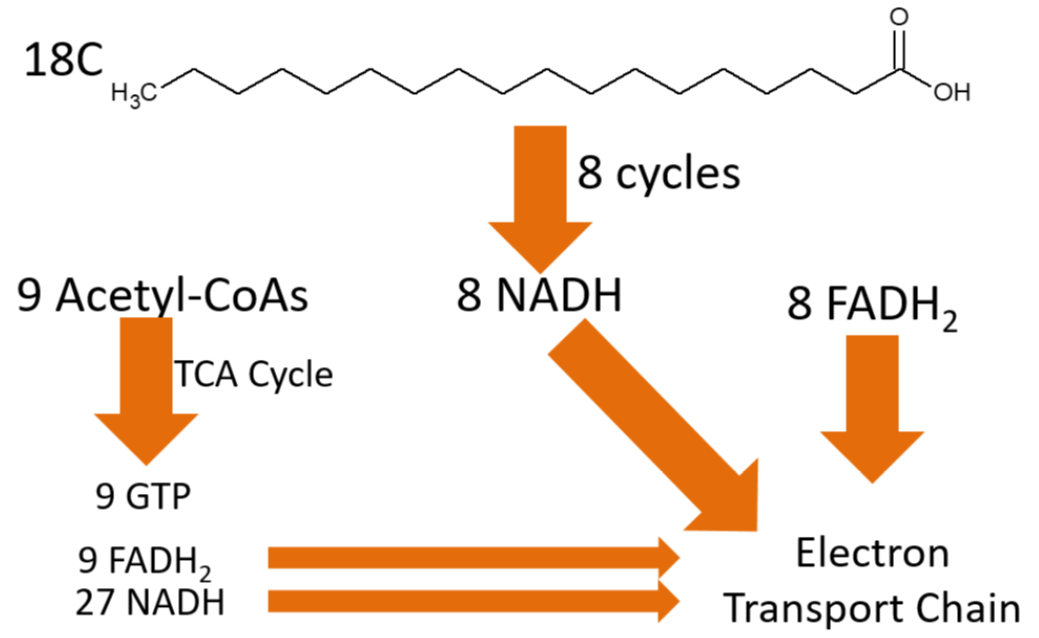
Adding up the \(\ce{NADH}\) and FADH2, the electron transport chain ATP production from beta-oxidation and the citric acid cycle looks like this:
\(\ce{NADH}\)
8 (beta-oxidation) + 27 (TCA) = 35 \(\ce{NADH}\) X 2.5 ATP/\(\ce{NADH}\) = 87.5 ATP
\(\ce{FADH2}\)
8 (beta-oxidation) + 9 (TCA) = 17 \(\ce{FADH2}\) X 1.5 ATP/\(\ce{FADH2}\) = 25.5 ATP
GTP
9 GTP = 9 ATP
Total ATP from complete oxidation of an 18 carbon fatty acid:
87.5 + 25.5 + 9 = 122 ATP
Subtract 2 ATP (ATP-->AMP) required for activation of the fatty acid:
122-2 = 120 Net ATP
Compared to glucose (32 ATP) you can see that there is far more energy stored in a fatty acid. This is because fatty acids are in a more reduced form and thus, they yield 9 kcal/g instead of 4 kcal/g like carbohydrates5.
The following animation reviews lipolysis and beta-oxidation.
Web Link
Query \(\PageIndex{4}\)
Query \(\PageIndex{5}\)
De novo Lipogenesis (Fatty Acid Synthesis)
De novo in Latin means "from the beginning." Thus, de novo lipogenesis is the synthesis of fatty acids, beginning with acetyl-\(\ce{CoA}\). Acetyl-\(\ce{CoA}\) has to first move out of the mitochondria, where it is then converted to malonyl-\(\ce{CoA}\) (3 carbons). Malonyl-\(\ce{CoA}\) then is combined with another acetyl-\(\ce{CoA}\) to form a 4 carbon fatty acid (1 carbon is given off as \(\ce{CO2}\)). The addition of 2 carbons is repeated through a similar process 7 times to produce a 16 carbon fatty acid6.

Most fatty acids synthesized will be esterified into triglycerides for storage.
Query \(\PageIndex{6}\)
Ketone Body Synthesis
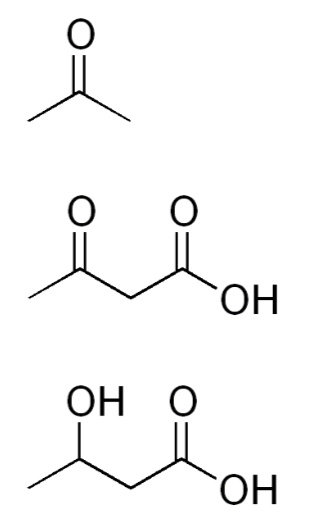
In cases where there is not enough glucose available for the brain (very low carbohydrate diets, starvation), the liver can use acetyl-\(\ce{CoA}\), primarily from fatty acids (but also certain amino acids), to synthesize ketone bodies (ketogenesis). The structures of the three ketone bodies; acetone, acetoacetic acid, and beta-hydroxybutyric acid, are shown below.
After they are synthesized in the liver, ketone bodies are released into circulation where they can travel to the brain. The brain converts the ketone bodies to acetyl-\(\ce{CoA}\) that can then enter the citric acid cycle for ATP production, as shown below.
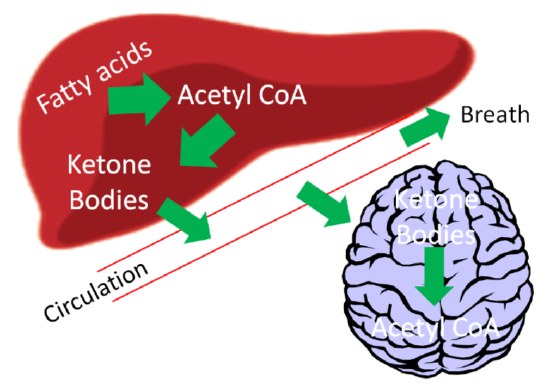
If there are high levels of ketones secreted, it results in a condition known as ketosis or ketoacidosis. The high level of ketones in the blood decreases the blood’s pH, meaning it becomes more acidic. It is debatable whether mild ketoacidosis is harmful, but severe ketoacidosis can be lethal. One symptom of this condition is fruity or sweet smelling breath, which is due to increased acetone exhalation.
Query \(\PageIndex{7}\)
Query \(\PageIndex{8}\)
Cholesterol Synthesis
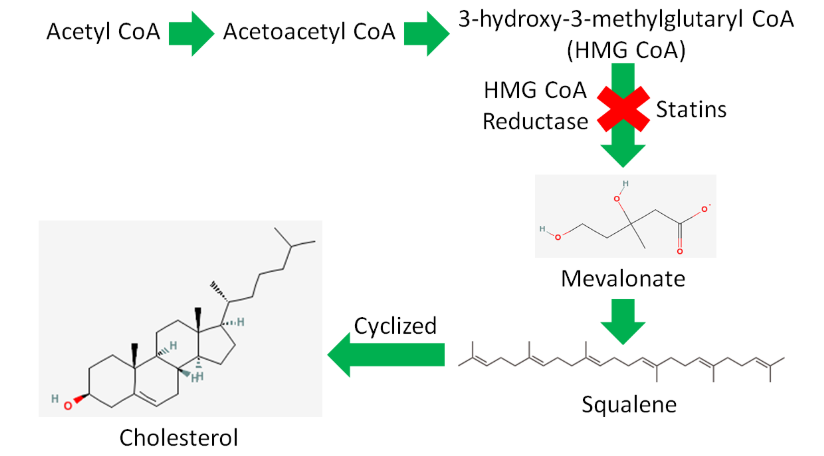
Acetyl-\(\ce{CoA}\) is also used to synthesize cholesterol. As shown below, there are a large number of reactions and enzymes involved in cholesterol synthesis.

Simplifying this, acetyl-\(\ce{CoA}\) is converted to acetoacetyl-\(\ce{CoA}\) (4 carbons) before forming 3-hydroxy-3-methylglutaryl-\(\ce{CoA}\) (HMG-\(\ce{CoA}\)). HMG-\(\ce{CoA}\) is converted to mevalonate by the enzyme HMG-\(\ce{CoA}\) reductase. This enzyme is important because it is the rate-limiting enzyme in cholesterol synthesis.
A rate-limiting enzyme is like a bottleneck in a highway, as shown below, that determines the flow of traffic past it.

Query \(\PageIndex{9}\)
Rate-limiting enzymes limit the rate at which a metabolic pathway proceeds. The pharmaceutical industry has taken advantage of this knowledge to lower people's LDL levels with drugs known as statins. These drugs inhibit HMG-\(\ce{CoA}\) reductase and thus decrease cholesterol synthesis. Less cholesterol leads to lower LDL levels, and hopefully a lower risk of cardiovascular disease.
The brand name of the statins approved for use in the US are13:
- Lipitor
- Lescol
- Mevacor
- Pravachol
- Crestor
- Zocor
- Livalo
The cholesterol guidelines have changed dramatically from the previous focus on LDL and HDL target levels. Now statins are prescribed at set therapeutic doses based on assessed cardiovascular risk rather than based off LDL and HDL target levels. It is also recommended that only statins that have been shown to decrease cardiovascular disease risk be used, some have only been shown to improve LDL/HDL levels. The link below is to the online calculator that can be used to estimate an individual's risk.
The body synthesizes approximately 1 g/day, whereas it is recommended that we consume less than 0.3 g/day. A number of tissues synthesize cholesterol, with the liver accounting for ~20% of synthesis. The intestine is believed to be the most active among the other tissues that are responsible for the other 80% of cholesterol synthesis1.
Query \(\PageIndex{10}\)
References
- Byrd-Bredbenner C, Moe G, Beshgetoor D, Berning J. (2009) Wardlaw's perspectives in nutrition. New York, NY: McGraw-Hill.
- en.Wikipedia.org/wiki/File:Ca..._structure.png
- simple.Wikipedia.org/wiki/Mi..._en_(edit).svg
- en.Wikipedia.org/wiki/Carnit...ial_matrix.svg
- Berg JM, Tymoczko JL, Stryer L. (2002) Biochemistry. New York, NY: W.H. Freeman and Company.
- Gropper SS, Smith JL, Groff JL. (2008) Advanced nutrition and human metabolism. Belmont, CA: Wadsworth Publishing.
- en.Wikipedia.org/wiki/Mitochondrion
- en.Wikipedia.org/wiki/File:Ketone_bodies.png
- commons.wikimedia.org/wiki/File:Liver.svg
- en.Wikipedia.org/wiki/Statin...se_pathway.png
- en.Wikipedia.org/wiki/File:Squalene.svg
- en.Wikipedia.org/wiki/File:Bottleneck.svg
- http://www.medicinenet.com/statins/page3.htm
- Gropper SS, Smith JL, Groff JL. (2008) Advanced nutrition and human metabolism. Belmont, CA: Wadsworth Publishing.


