1.7: Interpreting Research
- Page ID
- 40930
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Now that you are familiar with the different forms of nutrition research, the next step is understanding how to interpret and synthesize the information. To synthesize information on a certain topic, the two most popular methods are meta-analyses and systematic literature reviews. While there are differences between these two methods, they are similar in that they aim to draw a conclusion from the body of research evidence rather than from one study. We will focus on systematic literature reviews because of their rising popularity in biomedical literature.
A systematic literature review does what the name implies, it systematically reviews the literature related to a certain research question. For example, the research question might be, "Does chocolate decrease blood pressure?" There is a method for performing the review established ahead of time that details answers to questions such as:
- How will journal articles be identified?
- Using what databases?
- What search terms will be used?
The end product is a conclusion based on the evidence in the identified journal articles. The figure below illustrates how a systematic literature review identifies articles to utilize.

The video below, by the Cochrane Collaboration that performs many systematic literature reviews, describes what systematic literature are.
Because they synthesize the findings from multiple trials or studies, systematic literature reviews are considered the highest level of nutrition research evidence and are therefore shown at the top of the strength of nutrition research pyramid below. In vitro studies are shown at the bottom of the triangle because they are the weakest form of evidence overall. It should be pointed out that most systematic literature reviews only consider epidemiological studies and clinical trials and do not include animal studies or in vitro evidence. While these studies are of less strength, they should still be considered.

There are a couple of other factors to consider relating to the different forms of research. First, epidemiological studies cannot show causality (i.e. smoking causes lung cancer), but instead identify relationships or associations (i.e. smoking is associated with lung cancer occurrence). Clinical trials/human studies are the best form of primary research because their findings should be directly translatable to patients. So why use other forms of research? The description below should help explain why the other forms of research are also important.
In general, a certain sequence of studies in nutrition research is often followed as shown in the figure below.
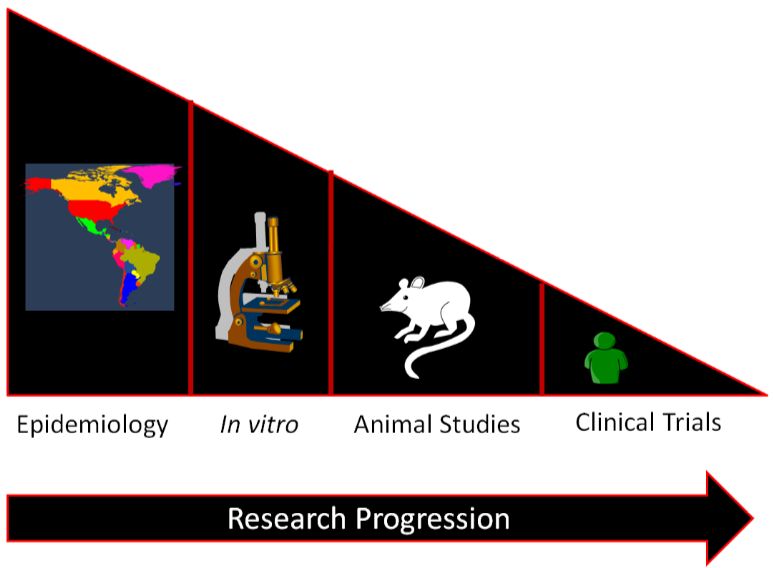
Epidemiological studies find relationships between food/food components and a specific health outcome. This relationship is then investigated by in vitro studies and then, some of the most promising move to animal studies. Then the most promising and safe food/food components are moved into clinical trials/human studies. If it is an individual compound, there will be smaller trials designed to see if the compound is safe before it is moved into larger clinical trials to determine whether the food/food component results in beneficial health outcomes. The overall effect of this process is to select the most promising and safe food/food components for the clinical trials/human studies. This allows time and money to be used more efficiently, because while clinical trials/human studies are the best form of research, they are also normally the most expensive and time-consuming.
Researchers nevertheless have been tempted to skip directly to clinical trials in the past rather than following the research progression. To illustrate what happens in these situations, the following describes a couple of examples when "normal" research progression hasn't been followed.
Query \(\PageIndex{1}\)
Query \(\PageIndex{2}\)
Query \(\PageIndex{3}\)
Beta-Carotene and Lung Cancer
In the early 1980s there was a lot of excitement among researchers over the epidemiological evidence showing that higher dietary consumption of the carotenoid, beta-carotene, decreased lung cancer risk1. By the mid 1980’s, two large, randomized, double-blind placebo-controlled trials began to determine whether high-dose beta-carotene supplementation (far higher than dietary intake) could decrease lung cancer incidence in high-risk populations before in vitro or animal studies had investigated this relationship. The research community was shocked when these two studies were terminated early in the mid 1990s because of significant increases in lung cancer incidence among smokers receiving beta-carotene supplements2,3. In vitro and animal studies completed after the clinical trial found that as beta-carotene intake shifts from normal dietary levels to high, supplement-type levels, the effect on lung cancer development also shifts from beneficial to detrimental in combination with smoke or carcinogen exposure4. Thus, if the normal research progression had been followed, it is likely that a lower dose of beta-carotene would have been used or trials wouldn't have been undertaken at all.
Selenium, Vitamin E, and Prostate Cancer
Another example of when the research progression was not followed was the relationship between selenium, vitamin E, and prostate cancer. Two clinical trials had found secondary outcomes that suggested that selenium and vitamin E supplementation may decrease prostate cancer risk 5,6. A secondary outcome is one that was not the primary outcome that the clinical trial was designed to find, thus they need to be interpreted with some caution. Rather than examining the relationship using in vitro and animal studies, a clinical trial was undertaken to determine if selenium and vitamin E supplementation alone, or in combination, could decrease the development of prostate cancer incidence7. This clinical trial was also terminated early because of a nonsignificant increase in prostate cancer in those receiving vitamin E8, and a nonsignificant increase in diabetes among those receiving selenium9. In vitro studies and animal models performed since the clinical trial was undertaken and after its termination suggest that vitamin E is not effective and that another form of selenium could have possibly been more effective10.
Query \(\PageIndex{4}\)
In addition to the lessons learned about the sequence of research in nutrition, these studies add to growing evidence that suggests that single-agent interventions, even in combination, may not be an effective strategy for improving health. The common nutrition research approach was, after epidemiology finds an association or relationship, is to use in vitro and animal studies to identify the specific compound in a certain food that is responsible. This has been termed the reductionist approach because it takes something complex (food) and reduces it down to its simpler components. However, there is growing evidence that this may be a flawed approach. Some nutrition researchers feel that more focus should be on the food itself, rather than trying to discern exactly what is responsible for the beneficial health outcomes. Because it may not be one or 2 compounds alone that are responsible for the effect, it might be difficult to determine from the multitude of nutrients in foods which are responsible for the beneficial effect. This will mean changes in the overall research approach, especially at the human intervention studies/clinical trials level, because in most cases there is not a way to give a “placebo food”11,12.
Query \(\PageIndex{5}\)
References
- Peto R, Doll R, Buckley JD, Sporn MB. Can dietary beta-carotene materially reduce human cancer rates? Nature 290, 201-208, 1981.
- The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. the alpha-tocopherol, beta carotene cancer prevention study group. N Engl J Med. 330, 1029-1035, 1994.
- Goodman GE, Thornquist MD, Balmes J, Cullen MR, Meyskens FL,Jr., Omenn GS, et al. The beta-carotene and retinol efficacy trial: Incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping beta-carotene and retinol supplements. J Natl Cancer Inst 96, 1743-1750, 2004.
- Lindshield BL, Erdman JW. (2006) Carotenoids. In: Bowman BA, Russell RM, editors. Present Knowledge in Nutrition. Washington, D.C.: International Life Sciences Institute. pp. 184-197.
- Clark LC, Dalkin B, Krongrad A, Combs GF,Jr., Turnbull BW, Slate EH, et al. Decreased incidence of prostate cancer with selenium supplementation: Results of a double-blind cancer prevention trial. Br J Urol 81, 730-734, 1998.
- Heinonen OP, Albanes D, Virtamo J, Taylor PR, Huttunen JK, Hartman AM, et al. Prostate cancer and supplementation with alpha-tocopherol and beta-carotene: Incidence and mortality in a controlled trial. J Natl Cancer Inst 90, 440-446, 1998.
- Lippman SM, Goodman PJ, Klein EA, Parnes HL, Thompson IM,Jr., Kristal AR, et al. Designing the selenium and vitamin E cancer prevention trial (SELECT). J Natl Cancer Inst 97, 94-102, 2005.
- Klein EA, Thompson IM, Tangen CM, Crowley JJ, Lucia MS, Goodman PH, Minasian LM, Ford LG, Parnes HL, Gaziano JM, Karp DD, Lieber MM, Walther PJ, Klotz L, Parsons JK, Chin JL, Darke AK, Lippman SM, Goodman GE, Meysken FL, Baker LH. Vitamin E and the Risk of Prostate Cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 306, 1549-1556, 2011.
- Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The selenium and vitamin E cancer prevention trial (SELECT). JAMA 301, 39-51, 2009.
- Lindshield BL, Ford NA, Canene-Adams K., Diamond AM, Wallig MA, Erdman JW Jr. Selenium, but not lycopene or vitamin E, decreases growth of transplantable Dunning R3327-H rat prostate tumors. PLoS One. 2010.
- Gann PH, Khachik F. Tomatoes or lycopene versus prostate cancer: Is evolution anti-reductionist? J Natl Cancer Inst 95, 1563-1565, 2003.
- Gann PH. Randomized trials of antioxidant supplementation for cancer prevention: First bias, now chance--next, cause. JAMA 301, 102-103, 2009.


