12.6: Vitamin A
- Page ID
- 40998
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)There are 3 forms of vitamin A (retinol, retinal, and retinoic acid) that collectively are known as retinoids. Retinol is the alcohol (\(\ce{OH}\)) form, retinal is the aldehyde (\(\ce{COH}\)) form, and retinoic acid is the carboxylic acid (\(\ce{COOH}\)) form, as shown in the figure below (areas of difference are indicated by red).

Among these different retinoids, retinol and retinal are fairly interchangeable. Either form is readily converted to the other. However, only retinal is used to form retinoic acid, and this is a one-way reaction. Thus, once retinoic acid is formed it can't be converted back to retinal, as shown in the figure below.

Query \(\PageIndex{1}\)
There are 2 primary dietary sources of vitamin A:
- Retinyl/retinol esters (Animal Products)
- Provitamin A Carotenoids (Plants)
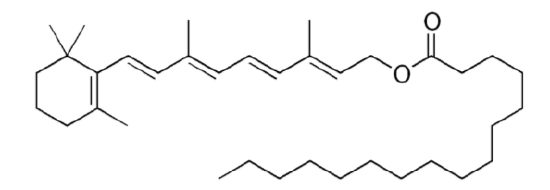
Preformed vitamin A means that the compound is a retinoid. Preformed vitamin A is only found in animal products (carrots are not a good source of preformed vitamin A). Most retinol in animal products is esterified, or has a fatty acid added to it, to form retinyl esters (aka retinol esters). The most common retinyl ester is retinyl palmitate (retinol + the fatty acid palmitate) whose structure is shown below.
Provitamin A is a compound that can be converted to vitamin A in the body, but currently is not in vitamin A form. The next section will talk about carotenoids, some of which are provitamin A compounds.
International units are also used for vitamin A, such that:
1 IU = 0.3 ug retinol or 0.6 ug beta-carotene
Query \(\PageIndex{2}\)
Query \(\PageIndex{3}\)
Carotenoids
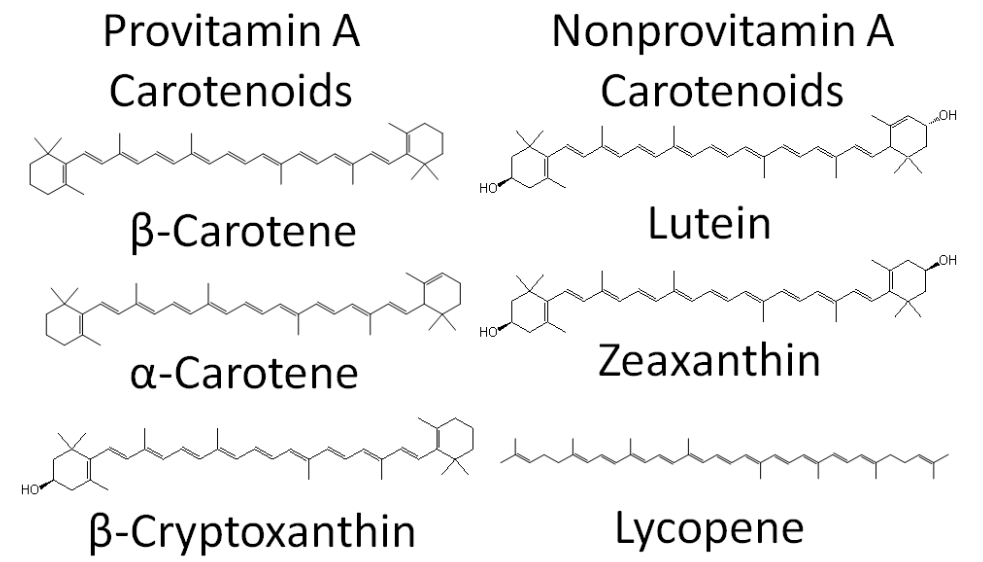
Carotenoids are 40-carbon compounds that are found throughout nature. Animals do not produce carotenoids, thus any found in animals (including people) come from consumed plants or microorganisms. There are more than 600 natural carotenoids. However, the 6 main ones found in the diet and in the body are3:
- Beta-carotene
- Alpha-carotene
- Beta-cryptoxanthin
- Lutein
- Zeaxanthin
- Lycopene
Many carotenoids are pigments, meaning they are colored. The table below gives the color of some of these carotenoids, as well as some food sources.
| Carotenoid | Color | Food Sources |
|---|---|---|
| Beta-carotene/Alpha-carotene | Orange | Carrots, Sweet Potatoes, Leafy Greens |
| Lycopene | Red | Tomatoes, Watermelon, Pink Grapefruit |
| Lutein/Zeaxanthin | Yellow | Kale, Corn, Egg Yolks, Spinach |
*Beta-cryptoxanthin is orange in color
Query \(\PageIndex{4}\)
Query \(\PageIndex{5}\)
Carotenoids can be further classified as provitamin A or non-provitamin A. Provitamin A carotenoids are those that can be cleaved to form retinal, while the non-provitamin A carotenoids cannot. The structure and classification of the 6 major carotenoids are shown below.
Query \(\PageIndex{6}\)
After provitamin A carotenoids are taken up into the enterocyte, some are cleaved to form retinal. In the case of symmetrical beta-carotene, it is cleaved in the center to form 2 retinal molecules as shown below.
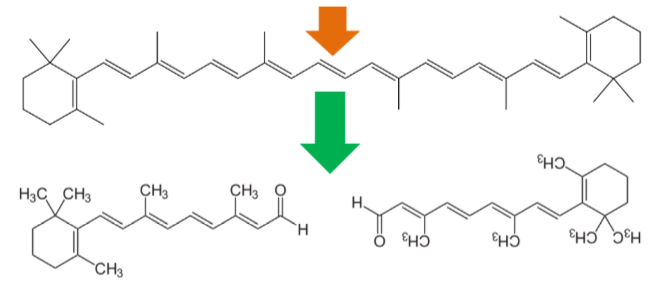
Alpha-carotene and beta-cryptoxanthin are asymmetrical, thus they can be used to form only 1 retinal.
To help account for the fact that retinol can be made from carotenoids, the DRI committee made retinol activity equivalents (RAE) that take into account the bioavailability and bioconversion of provitamin A carotenoids.
1 ug RAE
= 1 ug of retinol
= 2 ug of supplemental beta-carotene
= 12 ug of dietary beta-carotene
= 24 ug of alpha-carotene or beta-cryptoxanthin5
Supplemental beta-carotene is much more bioavailable than dietary beta-carotene found in a natural matrix within plant food components. As a result, you need to consume 6 times more dietary beta carotene to have the same RAE value as supplemental beta-carotene. The RAE difference between the provitamin A carotenoids is due to beta-carotene being cleaved to form 2 retinals, where alpha-carotene and beta-cryptoxanthin are cleaved 1 retinal. As a result, twice as much dietary alpha-carotene and beta-cryptoxanthin needs to be consumed to have the same RAE value as dietary beta-carotene.
Query \(\PageIndex{7}\)
Vitamin A Uptake, Absorption, Transport & Storage
The uptake, absorption, transport, and storage of vitamin A and carotenoids are summarized in the figure below.
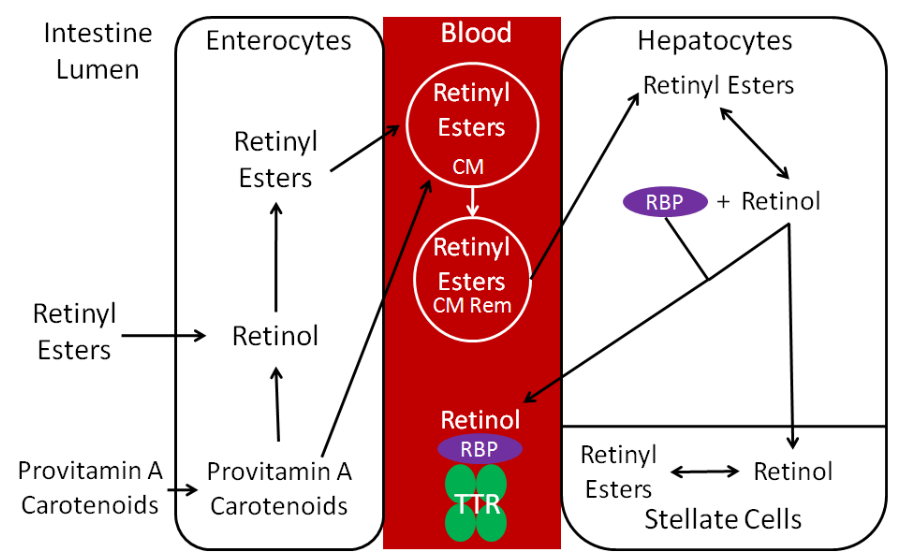
Esters are removed by esterases so that free retinol can be taken up into the enterocyte. Preformed vitamin A is highly bioavailable (70-90%) if consumed with some fat2. Carotenoids have a much lower bioavailability, which varies based on the carotenoid and matrix it is in when consumed. Once provitamin A carotenoids are taken up into the enterocytes, they are: (1) cleaved to retinal and then converted to retinol or (2) absorbed intact and incorporated into chylomicrons.
Retinol in the enterocyte is esterified, forming retinyl esters. Retinyl esters are packaged into chylomicrons (CM) and enter the lymph system. Once the chylomicrons reach circulation, triglycerides are cleaved off to form chylomicron remnants (CM Rem). These are taken up by hepatocytes, where the retinyl esters are de-esterified to form retinol. The liver is the major storage site of vitamin A. For storage, the retinol will be transported from hepatocytes to stellate cells and converted back to retinyl esters, the storage form of vitamin A.
If vitamin A is needed to be released into circulation, retinol will combine with retinol binding protein (RBP). Retinol + RBP are then bound to a large transport protein, transthyretin (TTR). It is believed that retinol + RBP would be filtered out by the kidney and excreted in urine if it was not bound to TTR6.
After it is further metabolized, 60% of vitamin A is excreted in the urine, 40% in feces7.
Query \(\PageIndex{8}\)
Vitamin A Nuclear Receptors
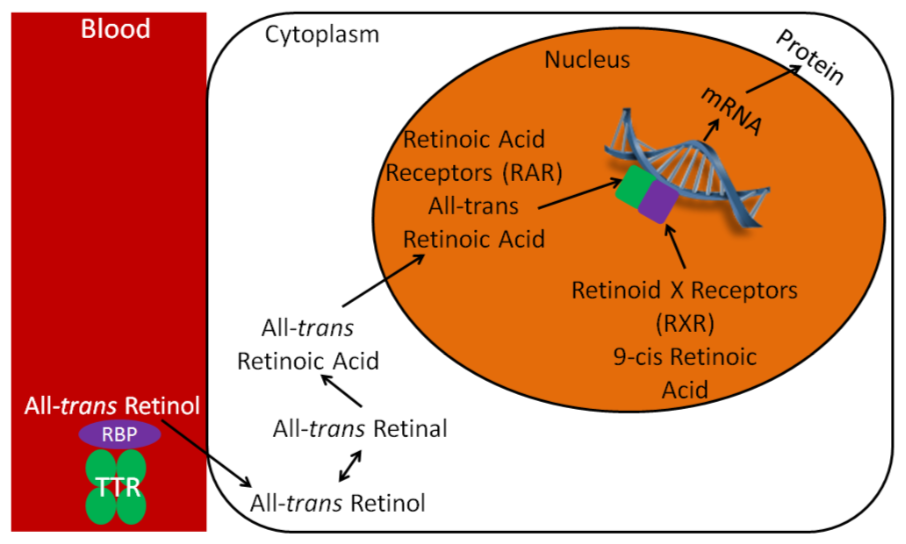
Vitamin A, like vitamin D, has a nuclear receptor. Vitamin A technically has two nuclear receptors: retinoic acid receptors (RARs) and retinoid X receptors (RXRs). Vitamin A, like polyunsaturated fatty acids, can be found in trans and cis forms, depending on the conformation of its double bonds. The ligand for RARs is all-trans-retinoic acid, and the ligand for RXRs is 9-cis retinoic acid.
As shown in the figure below, all-trans retinol is brought to the cell by RBP and TTR. All-trans retinol is converted to all-trans-retinal, and then to all-trans-retinoic acid. RAR and RXR are paired, or dimerized, on the retinoic acid response element (RARE) in the promoter region of target genes. The binding of all-trans retinoic acid to RAR causes the transcription and ultimately the translation of target proteins. This is why retinoic acid is the active form of vitamin A because all-trans retinoic acid is the ligand for RARs, leading to many of the biologic effects attributed to vitamin A. RXR is primarily a partner receptor for other nuclear hormone receptors and can serve in this role even without its ligand. 9-cis retinoic acid is rarely found in physiologically notable concentrations, so it is role is not entirely clear.
Query \(\PageIndex{9}\)
Vitamin A Functions
Vitamin A has a number of important functions in the body.
Vision
The retina is the inner back lining of the eye that takes visual images and turns them into nerve signals that are sent to the brain to form the images that we "see", as shown in the following figure.
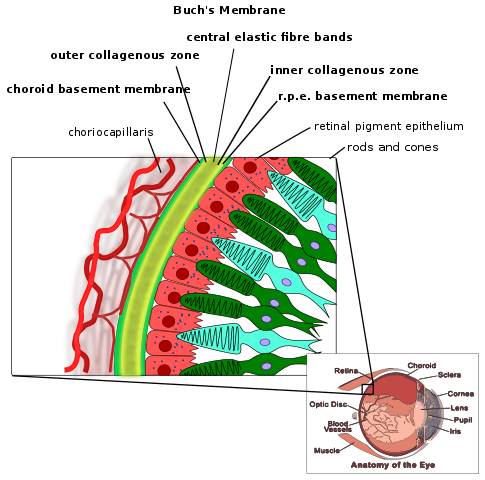
Inside the retina are the photoreceptor cells, rods and cones. Cones are responsible for color vision, while rods are important for seeing black and white. Within the rods, 11-cis retinal combines with the protein, opsin, to form rhodopsin. When light strikes rhodopsin, the compound splits into opsin and all-trans retinal. This sends a signal to your brain for us to “see” as illustrated below9.
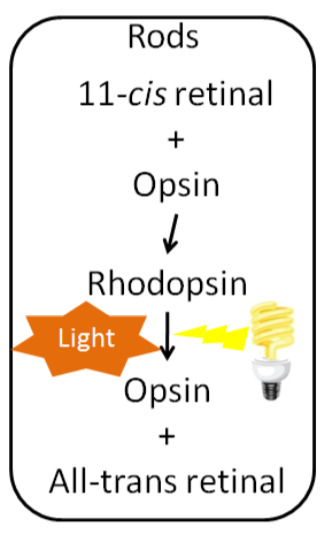
Most all-trans retinal is converted back to 11-cis retinal through a series of steps so it can continue to be used to form rhodopsin. However, this recycling is not 100% efficient. Vitamin A stores, or continued intake, is required to provide the 11-cis retinal needed to continue to form rhodopsin. Normally, our eyes adapt to darkness by increasing the amount of rhodopsin available so we can see under reduced light conditions9. If a person does not have enough rhodopsin he/she will become night blind, meaning his/her eyes do not adjust, or adjust very slowly, preventing he/she from seeing under limited light conditions. The picture below is an example of what night blindness looks like.

Query \(\PageIndex{10}\)
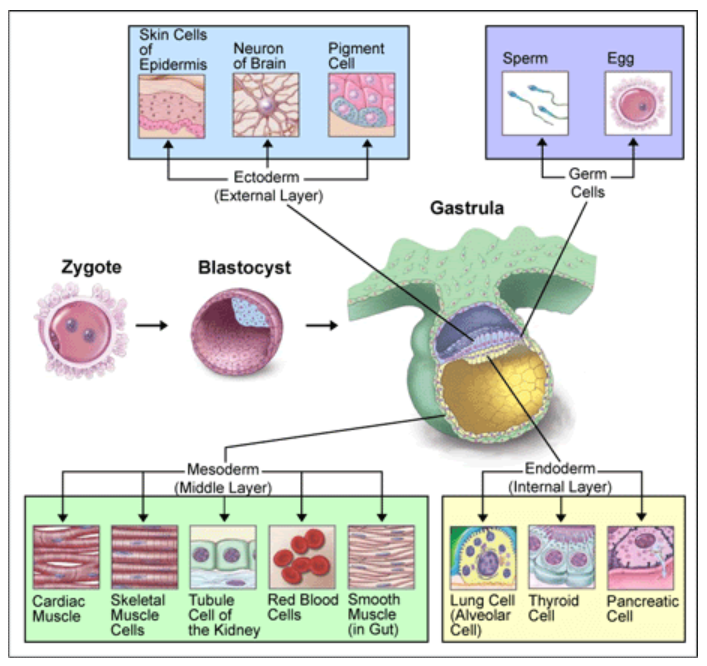
Cell Differentiation
Vitamin A, in particular retinoic acid, is important for cell differentiation, or the ability of stem cells to develop into specialized cells.
Other functions that vitamin A is important for include:
- Growth and development
- Reproduction
- Immune function
Query \(\PageIndex{11}\)
Vitamin A Deficiency & Toxicity
Vitamin A deficiency is rare in North America, but is a huge problem in developing countries. In many developing countries, they do not have a stable dietary source of retinoids or provitamin A carotenoids. The figure below gives you an idea of countries where vitamin A deficiency is a problem.
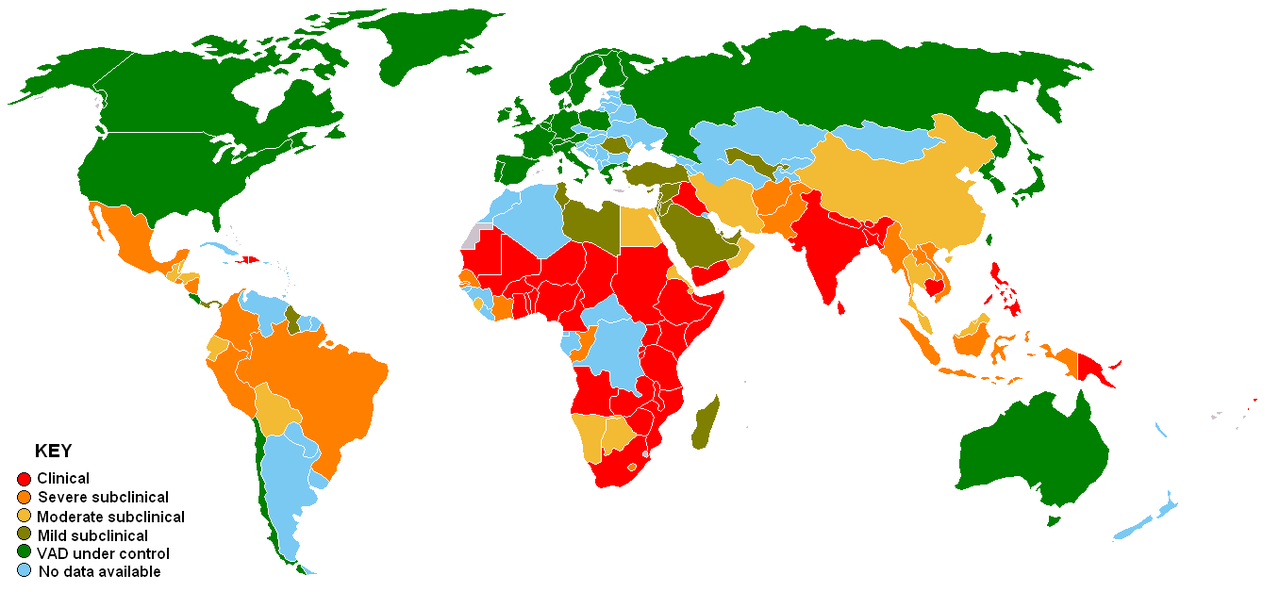
Often the earliest symptom of vitamin A deficiency is night blindness, due to the insufficient production of rhodopsin. The reason that this is the earliest symptom, is that circulating vitamin A (primary form is retinol) concentrations are homeostatically-controlled, meaning that they do not change until after vitamin A stores are exhausted (because stores are used to maintain circulating concentrations). This means that blood, serum, plasma measurements are going to appear normal until all stores are exhausted. As a result, determining or diagnosing someone as vitamin A deficient can be challenging. It also means that someone can be right on the brink of deficiency and an assessment of the status they would appear the same as someone who has adequate stores. There are further progressively worsening changes to the eye that occur during vitamin A deficiency, collectively referred to as xerophthalmia, which are shown in the link below.
Ultimately the person can become blind. Vitamin A deficiency is the leading cause of blindness in some parts of the world12.
Query \(\PageIndex{12}\)
Another symptom of vitamin A deficiency is hyperkeratosis. In this condition, cells overproduce the protein keratin, causing the skin to become rough and irritated, as shown in the link below12.
Web Link
One way to counter vitamin A deficiency in developing countries is for staple crops, like rice and corn, to contain beta-carotene. In the case of rice, Golden Rice was genetically modified to produce beta-carotene. A second generation of golden rice, known as Golden Rice 2, has now been developed. However, politics and regulations have prevented either from being used. This is described in the first link. The second link is a nice figure that details the progress towards Golden Rice being used. The third link describes the issues with how some research with it was conducted. The fourth link is an opinion article that gives you a feel for the opposition to its use.
Web Links
There are also corn varieties that have been bred (not genetically modified) to produce high amounts of beta-carotene, as described in the top link below. The second link describes how it has now been launched in the US.
Web Link
Query \(\PageIndex{13}\)
Vitamin A can be very toxic and can cause serious symptoms, such as blurred vision, liver abnormalities, skin disorders, and joint pain9,12. In addition, research has suggested that people who consume high levels of vitamin A are more prone to bone fractures12. Toxic levels of vitamin A are also teratogenic, which means they could cause birth defects.
This is important to keep in mind because a vitamin A derivative isotretinoin (13-cis retinoic acid) was the active ingredient in the oral acne medication, Accutane. Accutane was effective, as you can see in the video below.
However, due to the number of adverse events reported from its consumption, Accutane was recalled from the US market in 200913. Isotretinoin medications though are still commonly prescribed.
Retin-A is a topical product of all-trans-retinoic acid. Women of childbearing age need to exercise caution when using these products due to the risk of birth defects, should they become pregnant9. People should not consume huge doses of vitamin A expecting to get the same effects seen from these 2 medications14.
It is important to note that you cannot develop vitamin A toxicity from consuming too much beta-carotene or other provitamin A carotenoids. This is prevented because the enzyme cleavage of them is decreased in response to higher vitamin A status. Instead, a nontoxic condition known as carotenodermia occurs when large amounts of beta-carotene are consumed, where the accumulation of the carotenoid in the fat below the skin causes the skin to look orange, as shown in the link below. Excess lycopene consumption leads to a similar condition known as lycopenodermia, which appear more pink or orange.
Web Link
Query \(\PageIndex{14}\)
References
- Structures from Pubchem https://en.Wikipedia.org/wiki/Retinyl_palmitate#/media/File:Retinyl_palmitate.png
- https://en.Wikipedia.org/wiki/Retinyl_palmitate#/media/File:Retinyl_palmitate.png
- Lindshield BL, Erdman JW. (2006) Carotenoids. In: Bowman BA, Russell RM, editors. Present Knowledge in Nutrition. Washington, D.C.: International Life Sciences Institute. pp. 184-197.r
- https://s3-us-west-2.amazonaws.com/c...B141859F9A.png
- Anonymous. (2001) Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, D.C.: National Academies Press.
- Stipanuk MH. (2006) Biochemical, physiological, & molecular aspects of human nutrition. St. Louis, MO: Saunders Elsevier.Stipaunuk
- Gropper SS, Smith JL, Groff JL. (2008) Advanced nutrition and human metabolism. Belmont, CA: Wadsworth Publishing.
- https://commons.wikimedia.org/wiki/F...s_membrane.svg
- Byrd-Bredbenner C, Moe G, Beshgetoor D, Berning J. (2009) Wardlaw's perspectives in nutrition. New York, NY: McGraw-Hill.
- https://en.Wikipedia.org/wiki/Nyctalopia#/media/File:P360_Onderdendam_goed_nachtzicht_ns_nachtblind.jpg
- http://www.wikipremed.com/image.php?...mage_id=334000
- McGuire M, Beerman KA. (2011) Nutritional sciences: From fundamentals to food. Belmont, CA: Wadsworth Cengage Learning.
- http://www.drugwatch.com/accutane/
- Whitney E, Rolfes SR. (2011) Understanding nutrition. Belmont, CA: Wadsworth Cengage Learning.


