6.5: The Chemistry of Life’s Variety
- Page ID
- 56974
The potential number of variations of which the handful of amino acids are capable is mathematically huge. (Note that our whole language is built from just 26 letters, and that these same few units spell out many tongues.)
The rearrangements of these few symbols into such huge systems suggest how just 20 kinds of amino acids can be chained together to make a whole library of proteins. Since the chains of amino acids are so much longer than our words, the possibilities for variation in proteins are much greater. The proteins of life chemistry are usually at least 100 amino acids long, and they commonly range from 500 to 1,000.
Like our language, the proteins can be affected by the slightest change in sequence. Change the sequence of letters in words and you can change the meaning drastically—like going from baste to beats to beast. Thus, the same units serve radically different functions when they’re in a different order. Similar differences occur when you substitute one unit for another, as in six and sex.
Amino acid changes can have at least as much impact. Consider hemoglobin, the protein which carries oxygen in blood. It’s a chain of 146 amino acids. Change only the sixth amino acid (valine instead of glutamate) in the chain, and you have the defective hemoglobin that causes sickle cell anemia. Such tiny changes can, in many cases, spell the difference between sickness and health, between life and death.
Sickle cell anemia: An inherited disease caused by abnormal hemoglobin in red blood cells. Found mainly among those of African heritage.
The sequence of amino acids in a protein is known as its primary structure. But changes of the geometric arrangement of that chain can also alter the characteristics of the protein, multiplying possible variations almost to infinity.
As a very crude comparison, picture the amino acid chain of a protein as a thread. If we drop that thread, perhaps with a twirling motion, it can fall in a variety of patterns as it turns and twists and crosses over on itself.
The geometry of protein molecules is far more sophisticated. Remember our analogy of proteins as words, with the amino acids as letters. Suppose that these extremely long words could be written not only in straight lines but, without actually changing the sequence of the letters, in shapes, each of which had a different meaning, like this:
Or perhaps, using the name of the amino-acid phenylalanine:

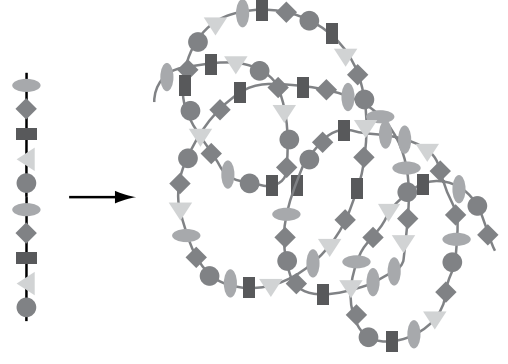
Recalling that these protein words are often 500 letters long, imagine the potential variety of shapes. But the variety doesn’t stop here. So far, we have considered the variety of two-dimensional geographic arrangement, using only the vertical and horizontal dimensions of this flat page. Imagine that, having written out our immensely long word in this wandering pattern, we could further change the meaning by crumpling the paper into a ball. Indeed, protein “words” are written in three dimensions (see Fig. 6-3).
Protein Shapes and Their Meaning in Life
How important is the shape of a protein? It’s extremely important because a protein’s shape is a key to its action. The hormone insulin (a protein) is an example. There are a few differences in amino acid sequence between human insulin and the insulin of other animals. Yet some animal insulin can work in the human body, because—despite the differences of sequence—the shape and cross-linkages are much the same as those of human insulin.
We’ve seen that a protein can change its characteristics when heated, agitated, or exposed to certain chemicals, as in the case of egg white. Such alterations are the result of changes in the shape—the three-dimensional structure—of the protein.
These changes in protein’s shape are known as denaturing (Fig. 6-4). Another example is the coagulation and color change of the blood and other “juices” on the surface of a broiling steak—also the result of the changing shape of protein.
The fact that denaturing a protein is a matter of changing its shape rather than changing its amino acid content has nutritional significance—denaturing protein by cooking doesn’t ordinarily lower its nutritional value.
Cooking, in fact, usually increases the protein value of food—especially plant foods—by making the food more digestible. The nutritional value of rice is markedly improved by cooking, since uncooked rice is quite indigestible. Increasing the digestibility of plants is especially important for the poor in developing countries, since plants are their major source of both the calories and the protein that are often deficient in their diet.
Denaturing inactivates proteins, including enzymes. A dim understanding of this has led many well-meaning “health-food” advocates to insist on raw foods, and to worry needlessly about some processing techniques. Enzymes in milk are denatured by pasteurizing, so some people insist on “raw” (unpasteurized) milk. For the same reason, some people demand wheat which has been stone-ground on the theory that more modern milling methods produce somewhat more heat and inactivate the enzymes of the grain.
But while they’re correct about the denaturing of enzymes, they fail to understand that the body has no use for a food’s enzymes—except as a bit of dietary protein. A food’s enzymes are treated by the body like all other food proteins—they’re broken down into amino acids by digestion and have no special value except as contributors of very small amounts of amino acids. One popular myth dear to weight-loss hucksters is that grapefruit enzymes can work some special magic to break down body fat. Untrue. Grapefruit enzymes are certainly useful—but only for grapefruit.
Cooks—and food companies—denature enzymes for good reason. By blanching (heating quickly and briefly) vegetables before freezing them, their enzymes are denatured and thus inactivated, retarding the deterioration of the vegetables. Typically, vegetables are blanched by a short immersion into boiling water—long enough to denature the enzymes, but brief enough to minimize other changes that might adversely affect the flavor, texture, and nutrient content of the vegetables.
Corn loses its sweetness as it ages because its sugar is turning into starch. By blanching the corn, the enzyme needed for this chemical reaction is inactivated, and the sweetness of corn is preserved. Anyone who has tried to add fresh pineapple to a gelatin concoction such as Jell-O knows that it doesn’t gel as it should. This mishap is avoided when canned pineapple is used because, in processing, the pineapple was heated, denaturing the enzyme in fresh pineapple that breaks down gelatin.
Proteins can also be denatured—and appear “cooked”—by acidifying their surrounding fluid. In the Latin American dish ceviche, raw seafood is marinated in seasoned lime juice and then served. The seafood appears to be cooked because the acid in the lime juice has denatured the protein of the seafood. We also see this acid effect in the curdling of milk proteins when the milk “goes sour,” or when we marinate beef in vinegar to make sauerbraten.
There can be problems in “cooking” food this way (marinating in lime juice) instead of using heat. In 1991, Peru had a deadly cholera epidemic when contaminated sewage tainted the seafood. Ceviche is a “fast food” popularly sold at food stands along the Peruvian coast. The mild acidity of lime juice doesn’t kill cholera organisms; thoroughly cooking the fish does.
Proteins in the body can be denatured by changes in acidity as well. The enzymes in blood and tissue fluids function optimally only when the acidity of these fluids is within its normally narrow range. This sensitivity is to be expected, since a change in acidity alters the shape of the enzymes and, thereby, alters their function.
Lactic acid is produced in muscles when oxygen and muscle glycogen become scarce during muscle activity, as in endurance events. This will be discussed in Chapter 11.
When athletes feel sudden fatigue (“hit the wall”) in endurance events, one explanation is that the lactic acid that has accumulated in their muscles has made the fluids in those muscles more acid—thereby, impairing their function.


