11.6: Aerobic Metabolism and the Mitochondria
- Page ID
- 57633
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
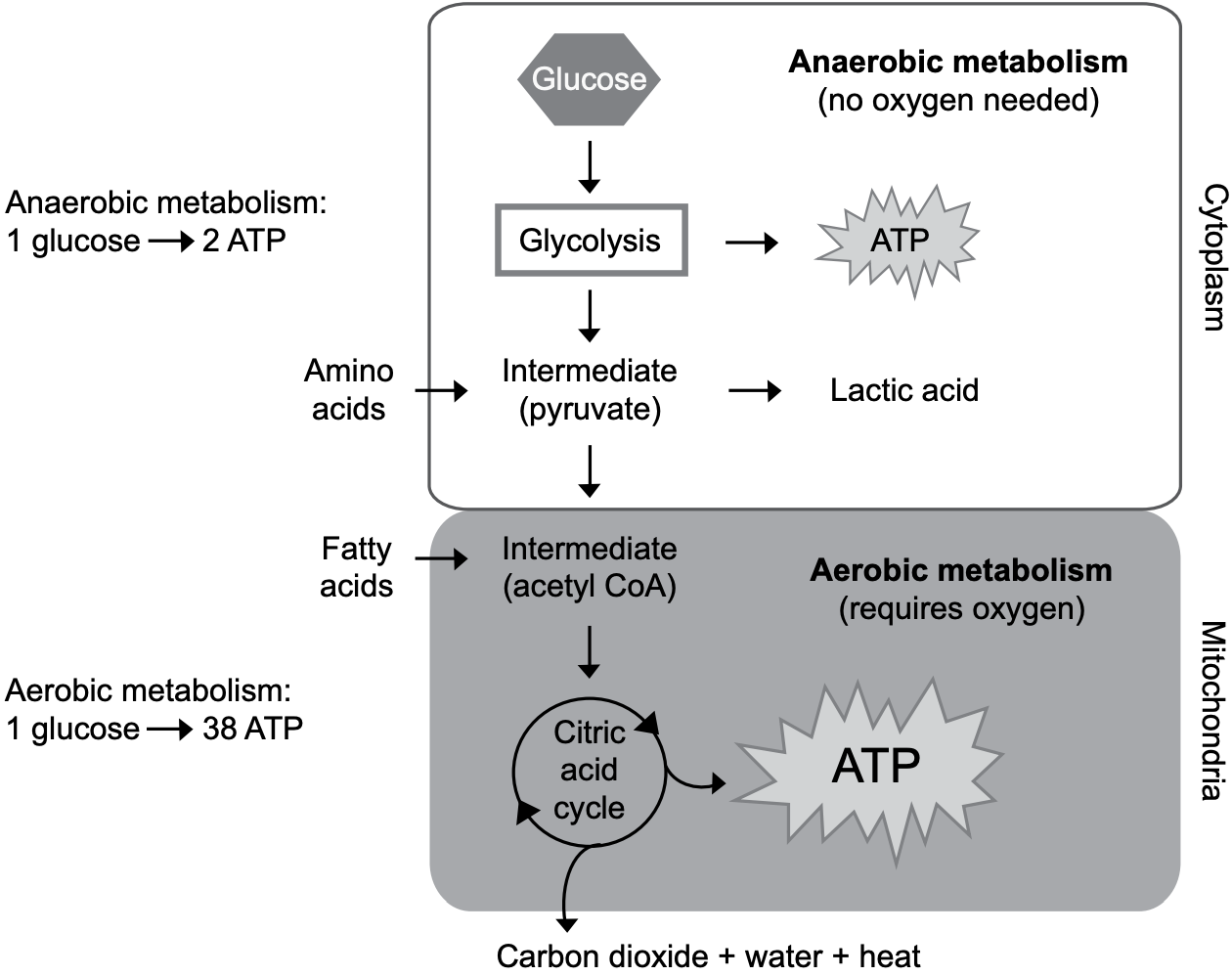
It doesn’t matter which nutrient—carbohydrate, protein, or fat—one begins with. Most of its energy is extracted in the same unifying process of aerobic energy metabolism that takes place in the mitochondria of cells (see Fig. 11-3).
Hans Krebs discovered this aerobic phase of metabolism, for which he received a Nobel Prize in 1953.
In a typical cell, there are 1,000 to 4,000 mitochondria. These mitochondria are the power plants of life. For it’s inside them that most of the energy from nutrients is released. The use of oxygen enables a net production of 38 molecules of ATP from one molecule of glucose. Without oxygen, net production is only 2 molecules of ATP. Because oxygen-requiring reactions (aerobic metabolism) take place only in the mitochondria, it’s clearly advantageous in terms of energy production for cells to contain mitochondria.
Each of these energy-providing nutrients has its own special path to reach this aerobic core of energy metabolism. Although the paths are intricate, the outlines are really quite simple.
Just remember that the energy of food is stored originally by photosynthesis—the joining of carbon dioxide (carbon, oxygen) and water (hydrogen, oxygen). And then more energy is stored as the resulting building blocks (compounds of carbon, hydrogen, and oxygen) are assembled into more and more complicated molecules which become the substance of our food.
Mitochondria as “Ancient Microbes”
It’s thought that mitochondria began as microbes that hundreds of millions of years ago permanently infected certain cells. Uninfected cells were unable to use oxygen and limited to the energy produced by glycolysis (anaerobic metabolism). When the supply of nutrients is limited, cells “infected” with mitochondria can make more ATP energy, giving them evolutionary advantage by enabling greater growth, movement, and synthesis of complex molecules.
True, other elements are added to the carbon-hydrogen-oxygen compounds along the way. Although these processes add complexity, the overall process remains simple. When we try to understand energy metabolism, we shouldn’t be distracted from the cell’s central purpose.
Fundamentally, the cell’s energy objective is a primitive one—as old as the first stirrings of life. It is, in effect, to reverse the process of chemical building. It is to tear apart the chemicals which make food, and keep tearing them apart, releasing the energy with which they were put together, until there’s nothing left but the original carbon dioxide and water.

In the aerobic phase of energy metabolism, this final resolution is achieved. All the stages of preparation which lead to this phase serve to filter out the substances that can’t participate, and end with only a small, single molecule (acetic acid) made of carbon, hydrogen, and oxygen.
In energy metabolism, all the energy-providing nutrients—carbohydrate, fat, protein—are broken down to the same small molecule (acetic acid).
It’s this little molecule—still holding much of the energy of the big molecules of food—which finally becomes carbon dioxide and water again, and yields food energy to the mitochondria and to life. Let’s see how carbohydrate, fat, and protein, eventually reach this point, starting with carbohydrate.
Coenzyme A and the Final Preparation of Glucose
Carbohydrates are absorbed from the intestine as single sugars, predominately glucose. (Other single sugars are, for the most part, immediately converted to glucose upon absorption.)
As we have seen, glucose is converted to pyruvate (see Fig. 11-2). Only one chemical step then remains to change the pyruvate into the acetic acid (carried as acetyl CoA) that goes into the aerobic phase of energy metabolism. (Acetic acid is more commonly known as vinegar.)
The conversion of pyruvate to acetic acid is straightforward, but the execution is intricate. It involves some large and complicated coenzymes that contain some vitamins as part of their structure. The process is worth looking at to see some of the ways in which our body needs vitamins.
First, the pyruvate combines with a coenzyme called thiamin pyrophosphate. From the name, we correctly surmise that the coenzyme includes thiamin (the B-vitamin missing in beriberi).
In a series of changes which follow, three more B-vitamins become involved. A coenzyme containing niacin (the B-vitamin missing in pellagra) and a coenzyme containing riboflavin (another B-vitamin) are also needed to convert pyruvate to acetic acid.
Finally, the acetic acid is attached to coenzyme A, which contains pantothenic acid (another B-vitamin), This acetic acid and coenzyme A combination is called acetyl CoA.
The end result of all this is that glucose is broken down to acetyl CoA, which is what enters the aerobic phase of metabolism (see Fig. 11-3). In other words, coenzyme A carries the acetic acid made from glucose into the final phase of energy metabolism—the aerobic, high-energy-yielding phase that converts acetic acid to carbon dioxide and water.
While the details of this aerobic core of energy metabolism are complex, the essence of what’s happening may be envisioned in a simple way.
Remember that when glucose is made from carbon dioxide and water by photosynthesis, oxygen is freed. If the process is to be reversed, the oxygen must be restored. This is why this release of energy from food is referred to as oxidation or aerobic (air-dependent) energy metabolism. Oxygen is restored to the carbons in acetic acid, forming carbon dioxide and water—and ample amounts of ATP energy (see Fig. 11-3).
How Fats Yield Their Energy
As discussed in Chapter 8, the fat in our fat stores and most of the fat in our diet are triglycerides. For use in energy production, the fatty acids in these triglycerides are let loose.
Like glucose, fatty acids are compounds of carbon, hydrogen, and oxygen. Fatty acids, however, have much less oxygen in their structure (see page 96: glucose C6H12O6, caproic acid C6H12O2). Compared to glucose, they can then join with more than twice as much oxygen to produce carbon dioxide and water. This is why fat (9 calories/ gram) has more than twice the energy potential of carbohydrate (4 cal/gm).
Fatty acids—whatever the length of their carbon chain—are broken down to acetic acid, and join Coenzyme A to become acetyl CoA. They thus enter aerobic metabolism, indistinguishable from the acetyl CoA that came from glucose (see Fig. 11-3).
For his part in discovering the chemical reactions that break down fatty acids to acetic acid, Feodor Lynen won a Nobel Prize in 1964.
It should be noted that energy isn’t released from fatty acids until after they enter aerobic metabolism as acetyl CoA. The energy-releasing metabolism of fatty acids doesn’t include glycolysis. The anaerobic release of energy by glycolysis is unique to carbohydrates (and a few amino acids). In other words, oxygen is essential for energy production from fatty acids.
How Amino Acids Enter Energy Metabolism
As we saw in Chapter 6, the amino acids that make up proteins are also built primarily from carbon, hydrogen, and oxygen. But they are characterized by the addition of nitrogen, in the form of the amino group (NH2).
In order to be used for energy, amino acids must first have their amino groups removed. As discussed in Chapter 7, these amino groups are a liability, since they can be converted to ammonia, which is toxic even in fairly low concentrations. To prevent this toxic effect, the amino groups are used to make urea, which can be tolerated at much higher concentrations (see Fig. 11-4). Then, the urea is excreted in the urine.
Once the amino acids are stripped down to their carbon-hydrogen-oxygen structure, they become suitable for the energy-releasing reactions of metabolism.
The carbon skeletons of amino acids, however, are a lot more varied than those of glucose or fatty acids. Depending on its structure, an amino acid is converted to either pyruvate or acetic acid (acetyl CoA). In doing so, the amino acids become indistinguishable from the pyruvate that came from glucose or from the acetyl CoA that came from glucose and fatty acids.
We see here a model of efficiency in the energy-releasing reactions of metabolism. All energy-providing nutrients funnel through acetyl CoA in releasing their energy while breaking down to carbon dioxide and water (see Fig. 11-3).

A coenzyme that contains vitamin B6 is needed for the conversion of amino acids to acetic acid (acetyl CoA).
Take note of the two waste products that result once the energy has been freed—carbon dioxide and water. The system has gone full circle. And the atoms which formed our food have not themselves been changed or used. The food molecules have merely served as energy carriers—with solar energy being used in plants to put and hold the atoms together, and energy being released in our cells when the atoms are separated again.


