10.4: Minerals Important for Blood Function and Renewal
- Page ID
- 23367
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- List the primary function of each of the minerals involved in metabolism.
- Summarize the roles of minerals important in blood function and renewal.
In a well-balanced diet, minerals are plentiful, and herbs are power-packed with minerals. Eat more plants, spice up your food, and drink herbal teas to obtain optimum mineral nutrition.

Iron
Iron is a trace mineral that we need small quantities every day. It is slightly water soluble and is a cation that exists in two states, +2 (ferrous) or +3 (ferric). Red blood cells contain the oxygen carrier protein hemoglobin. It is composed of four globular peptides, each containing a heme complex. In the center of each heme, lies iron (Figure 10.4.2). Eighty percent of the body's iron is bound to hemoglobin. In the muscle, iron is part of the oxygen-binding protein, myoglobin. Iron is a key component of hundreds of metabolic enzymes. Many of the proteins of the electron transport chain contain iron–sulfur clusters involved in the transfer of high-energy electrons and ultimately ATP synthesis. Iron is also involved in numerous metabolic reactions that take place mainly in the liver and detoxify harmful substances. Moreover, iron is required for DNA synthesis. The great majority of iron used in the body is that recycled from the continuous breakdown of red blood cells.
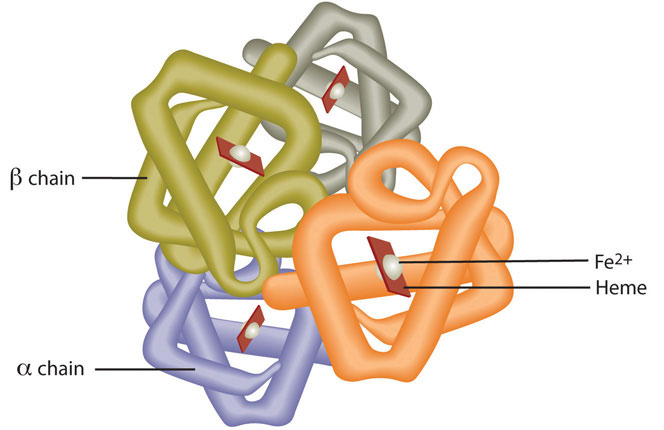
Figure \(\PageIndex{1}\): Hemoglobin is composed of four peptides. Each contains a heme group with iron in the center.
The iron in hemoglobin binds to oxygen in the capillaries of the lungs and transports it to cells where the oxygen is released (see "Video 10.5.1"). If iron levels are low hemoglobin is not synthesized in sufficient amounts and the oxygen-carrying capacity of red blood cells is reduced, resulting in anemia. When iron levels are low in the diet the small intestine more efficiently absorbs iron in an attempt to compensate for the low dietary intake, but this process cannot make up for the excessive loss of iron that occurs with chronic blood loss or low intake. Only 2-35% of the iron in your diet is absorbed. Once absorbed, the iron may be incorporated into hemoglobin or myoglobin to transport oxygen, or incorporated into other proteins, or stored in the liver, bone marrow or spleen. Ferritin is the molecule that stores iron in mucosal cells. When the iron is needed it is released from the ferritin and transported by transferrin to other cells for use. Once in the body, it is difficult to excrete iron but some iron will be lost when mucosal cells of the gut are shedded, a process that occurs about every 3-5 days.
Erythrocytes or RBCs circulate for about 120 days and then they are removed. When blood cells are decommissioned, the liver and spleen degrade the red blood cell and transferrin or recycles the iron back to the liver the bone marrow where red blood cells are made. The heme component is recycled into bile. Hepcidin, a hormone produced by the liver, regulates iron balance by inhibiting its release. A relatively small amount of iron is excreted when cells lining the small intestine and skin cells die and in blood loss, such as during menstrual bleeding. The lost iron must be replaced from dietary sources. Watch this video to view how hemoglobin in red blood cells transports oxygen to all cells in the body.
Video \(\PageIndex{1}\): Oxygen Transport. Watch this video to view how hemoglobin in red blood cells transports oxygen to all cells in the body.
Dietary iron comes in two forms, as heme iron or as nonheme iron. The bioavailability of iron is highly dependent on dietary sources and the form of iron. In animal-based foods, about 60 percent of iron is bound to hemoglobin, and heme iron is more bioavailable than nonheme iron. The other 40 percent of iron in animal-based foods is nonheme, which is the only iron source in plant-based foods. Some plants contain chemicals (such as phytate, oxalates, tannins, and polyphenols) that inhibit iron absorption. Although, eating fruits and vegetables rich in vitamin C at the same time as iron-containing foods markedly increases iron absorption. A review in the American Journal of Clinical Nutrition reports that in developed countries iron bioavailability from mixed diets ranges between 14 and 18 percent and that from vegetarian diets range between 5 and 12 percent.Centers for Disease Control and Prevention. “Iron and Iron Deficiency.” Accessed October 2, 2011. www.cdc.gov/nutrition/everyone/basics/vitamins/iron.html. Vegans are at higher risk for iron deficiency, but careful meal planning does prevent its development. Iron deficiency is the most common of all micronutrient deficiencies and will be explored in depth in Section 10.5 "Iron-Deficiency Anemia".
Zinc
Zinc is a trace mineral that is a cation and cofactor for over two hundred enzymes in the human body that play a direct role in RNA, DNA, and protein synthesis. Zinc also is a cofactor for enzymes involved in energy metabolism. It is important for wound healing, transport of vitamin A, taste perception, growth and development, and cofactor in genetic material and protein (zinc fingers).
As the result of its prominent roles in anabolic and energy metabolism, a zinc deficiency in infants and children blunts growth. The reliance of growth on adequate dietary zinc was discovered in the early 1960s in the Middle East where adolescent nutritional dwarfism was linked to diets containing high amounts of phytate. Cereal grains and some vegetables contain chemicals, one being phytate, which blocks the absorption of zinc and other minerals in the gut. It is estimated that half of the world’s population has a zinc-deficient diet.Prasad, Ananda. “Zinc deficiency.” BMJ 2003 February 22; 326(7386): 409–410. doi: 10.1136/bmj.326.7386.409. Accessed October 2, 2011. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1125304/?tool=pmcentrez. This is largely a consequence of the lack of red meat and seafood in the diet and reliance on cereal grains as the main dietary staple. In adults, severe zinc deficiency can cause hair loss, diarrhea, skin sores, taste changes, loss of appetite, impaired vision, impaired immune function, and weight loss. Zinc is a required cofactor for an enzyme that synthesizes the heme portion of hemoglobin and severely deficient zinc diets can result in anemia. People at risk of developing a zinc deficiency are people consuming excess iron, copper and/or dietary fiber, people that lack financial access to a nutrient adequate diet, the elderly, pregnant women and children. Zinc is rich in foods high in protein.
Copper
Copper is a trace mineral that is cation and exists in the +1 or +2 state. Like iron, copper assists in electron transfer in the electron-transport chain. Furthermore, copper is a cofactor of enzymes essential for iron absorption and transport, and hemoglobin synthesis. The other important function of copper is an antioxidant, which was also discussed in Chapter 8 "Nutrients Important As Antioxidants".
Symptoms of mild to moderate copper deficiency are rare. More severe copper deficiency can cause anemia from the lack of iron mobilization in the body for red blood cell synthesis. Other signs and symptoms include growth retardation in children and neurological problems because copper is a cofactor for an enzyme that synthesizes myelin, which surrounds many nerves. Groups at risk of developing a copper deficiency are people who consume too much zinc.
Menke's disease is a genetic disorder that causes a copper deficiency. The onset occurs typically during infancy and is characterized by sparse, kinky hair; failure to gain weight and grow at the expected rate (failure to thrive); and deterioration of the nervous system. Often these children do not live past age 3. Early treatment with copper may improve the prognosis in some affected individuals.
Individuals with the rare inherited disease, Wilson's disease, accumulate copper in the liver and brain. Excess copper intake
| Mineral | Function | Deficiency: Signs and Symptoms |
|---|---|---|
| Trace | ||
| Iron | Assists in energy production, DNA synthesis required for red blood cell function | Anemia: fatigue, paleness, faster heart rate |
| Zinc | Assists in energy production, protein, RNA and DNA synthesis; required for hemoglobin synthesis | Growth retardation in children, hair loss, diarrhea, skin sores, loss of appetite, weight loss |
| Copper | Assists in energy production, iron metabolism, cofactor for enzymes | Anemia: fatigue, paleness, faster heart rate |
Dietary Reference Intakes for Minerals and Dietary Sources
The RDA set by the IOM for minerals involved in metabolism are listed for adults in Table 10.4.2 "Dietary Reference Intakes and Food Sources for Minerals Important for Metabolism". The table also lists dietary sources for these micronutrients. The mineral content of foods is greatly affected by the soil from which it grew, and thus geographic location is the primary determinant of the mineral content of foods. For instance, iodine comes mostly from seawater so the greater the distance from the sea the lesser the iodine content in the soil.
| Mineral | RDA (mg/day) | Food Sources |
|---|---|---|
| Trace | ||
| Iron | 8 (males) |
Heme sources: Animal products such as meat, fish, and poultry Non-heme sources: fortified breakfast cereals, beans (tofu), spinach, peas, dried fruit, whole grains |
| 18 (females) | ||
| Zinc | 11 (males) | Red meat, poultry, seafood, fortified breakfast cereals, beans, nuts, whole grains |
| 8 (females) | ||
| Copper | 0.900 (males) | Whole grains, liver, legumes, seeds, cocoa, water if copper pipes |
| 0.900 (females) | ||
| * denotes Adequate Intake, **for ages 31–50 only | ||
Source: Institute of Medicine. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. January 9, 2001. www.iom.edu/Reports/2001/Dietary-Reference-Intakes-for-Vitamin-A-Vitamin-K-Arsenic-Boron-Chromium-Copper-Iodine-Iron-Manganese-Molybdenum-Nickel-Silicon-Vanadium-and-Zinc.aspx.
Bioavailability
Minerals are not as efficiently absorbed as most vitamins and so the bioavailability of minerals can be very low. Plant-based foods often contain factors, such as oxalate and phytate, that bind to minerals and inhibit their absorption. In general, minerals are better absorbed from animal-based foods. In most cases, if dietary intake of a particular mineral is increased, absorption will decrease. Some minerals influence the absorption of others. For instance, excess zinc in the diet can impair iron and copper absorption. Conversely, certain vitamins enhance mineral absorption. For example, vitamin C boosts iron absorption, and vitamin D boosts calcium and magnesium absorption. As is the case with vitamins, certain gastrointestinal disorders and diseases, such as Crohn’s disease and kidney disease, as well as the aging process, impair mineral absorption, putting people with malabsorption conditions and the elderly at higher risk for mineral deficiencies.
Key Takeaways
- Minerals cannot be broken down to release energy.
- Minerals are cofactors for hundreds of enzymes involved in metabolism.
- Iron especially, but also copper and zinc are critical for blood function and renewal.
- Minerals are not as efficiently absorbed as most vitamins and bioavailability can be very low.
Discussion Starters
- Discuss why “more is not always better,” especially how this saying pertains to micronutrient intake.
- Look up the chemical structure of phytate and explain how it inhibits the absorption of positively charged minerals.

