2.1: Carbohydrates - Simple Carbohydrates & Alternative Sweeteners
- Page ID
- 20968
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Carbohydrates have become surprisingly divisive. Some people swear by them, others swear against them. But it is important to understand that carbohydrates are a diverse group of compounds that have a multitude of effects in the body. Thus, trying to make blanket statements about carbohydrates is probably not a good idea.
Carbohydrates are named because they are hydrated (as in water, \(\ce{H2O}\)) carbon. Below is the formula showing how carbon dioxide (\(\ce{CO2}\)) and water (\(\ce{H2O}\)) are used to make carbohydrates (\(\ce{(CH2O)_{n}}\)) and molecular oxygen (\(\ce{O2}\)). The “\(\ce{n}\)” after the carbohydrate in the formula indicates that the chemical formula is repeated an unknown number of times, but that for every carbon and oxygen, there will always be two hydrogens.
\[\ce{nCO2 + nH2O \rightarrow (CH2O)_{n} + nO2}\]
Carbohydrates are produced by plants through a process known as photosynthesis. In this process, plants use the energy from photons of light to synthesize carbohydrates. The formula for this reaction looks like this:
\[\ce{6CO2 + 6H2O + Light \rightarrow C6H12O6 + 6O2} \]
There are many different types of carbohydrates as shown in the figure below. The first way that carbohydrates can be divided into simple, complex, and sugar alcohols. As the names imply, complex carbohydrates contain more sugar units, while simple carbohydrates contain either 1 or 2 sugars. In the next sections, you will learn more about the different forms of carbohydrates.
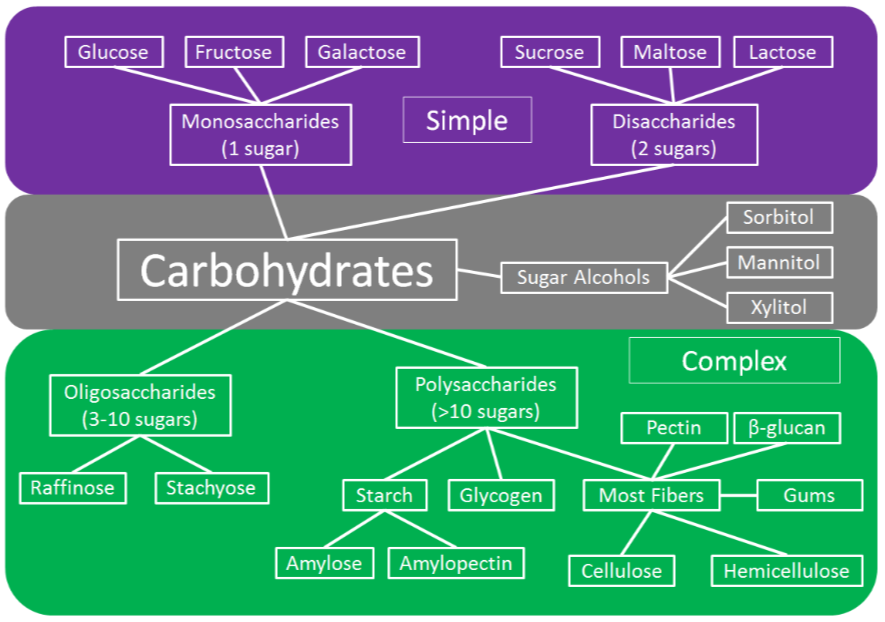
Simple Carbohydrates
As shown in the figure below, simple carbohydrates can be further divided into monosaccharides and disaccharides. Mono- means one, thus monosaccharides contain one sugar. Di- means two, thus disaccharides contain 2 sugar units. Refer to Figure \(\PageIndex{1}\) for a general overview of carbohydrates.
Monosaccharides
The 3 monosaccharides are: glucose, fructose and galactose. Notice that all are 6-carbon sugars (hexoses). However, fructose has a five member ring, while glucose and galactose have 6 member rings. Also notice that the only structural difference between glucose and galactose is the position of the hydroxyl (\(\ce{OH}\)) group that is shown in red.
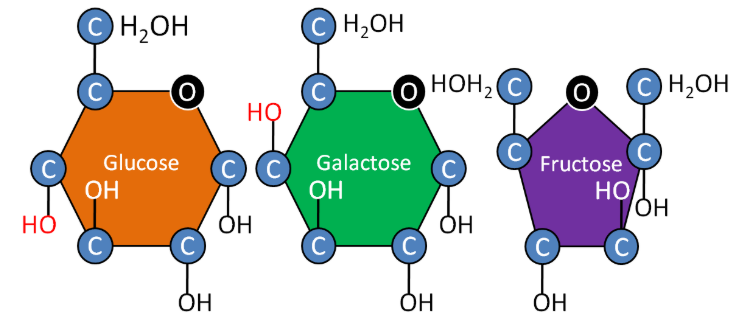
- Glucose - Product of photosynthesis, major source of energy in our bodies
- Galactose - Not normally found in nature alone, normally found in the disaccharide lactose
- Fructose - Sweetest, commonly found in fruits and used commercially in many beverages
Disaccharides
Disaccharides are produced from 2 monosaccharides. The commonly occurring disaccharides are:
- Maltose (glucose + glucose, aka malt sugar) - seldom found in foods, present in alcoholic beverages and barley
- Sucrose (glucose + fructose, aka table sugar) - only made by plants.
- Lactose (galactose + glucose, aka milk sugar) - primary milk sugar
The different disaccharides and their monosaccharides components are illustrated below.
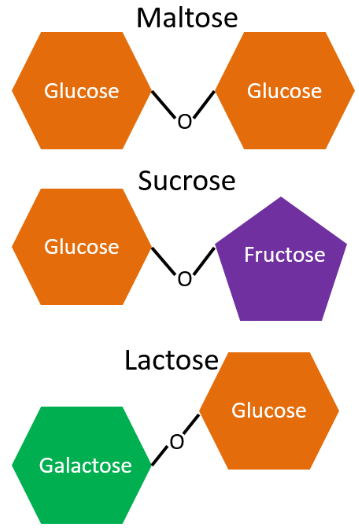
Notice the difference in the bond structures. Maltose and sucrose have alpha-bonds, which are depicted as v-shaped above. You might hear the term glycosidic used in some places to describe bonds between sugars. A glycoside is a sugar, so glycosidic is referring to a sugar bond. Lactose, on the other hand, contains a beta-bond. We need a special enzyme, lactase, to break this bond, and the absence of lactase activity leads to lactose intolerance.
High-Fructose Corn Syrup
Food manufacturers are always searching for cheaper ways to produce their food. One method that has been popular is the use of high-fructose corn syrup as an alternative to sucrose. High-fructose corn syrup contains either 42 or 55% fructose, with the rest being made up of glucose, meaning it is similar to sucrose in the amount of these monosaccharides it contains1. The major difference is that glucose and fructose are free in high-fructose corn syrup, rather than bonded together like in sucrose. Nevertheless, because the increase in high-fructose corn syrup consumption (see figure below) coincided with the increase in obesity in the U.S., there is a lot of controversy surrounding its use.
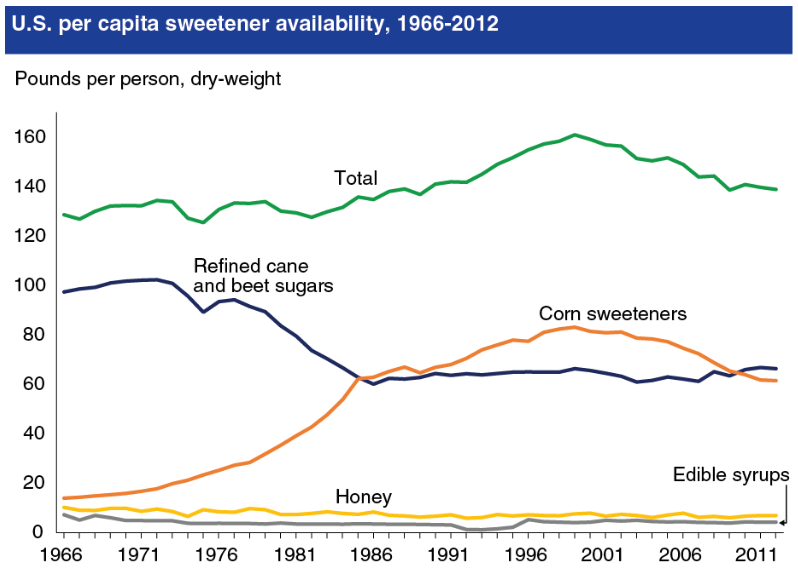
Opponents claim that high-fructose corn syrup is contributing to the rise in obesity rates. As a result, food products are being formulated with table sugar instead of high-fructose corn syrup and the fact that the product does not contain high-fructose corn syrup is being marketed or noted on packaging. Manufacturers tried to rebrand high-fructose corn syrup as "corn sugar" to get around the negative perception of the name. But the FDA rejected this request as described in the link below.
However, this concern with high-fructose corn syrup is not warranted given that fructose consumption is what ideally should be limited because it is metabolized differently than the other monosaccharides and may lead to the adverse effects that people are conflating with high-fructose corn syrup because of its name. High-fructose corn syrup provides about the same amount of fructose, and as you will learn later, we digest carbohydrates down to free monosaccharides before they are taken up and absorbed. Thus, what we get from consuming high-fructose corn syrup and sucrose is about the same amount of fructose. There is no reason to believe that there should be differences in outcomes given that what we get from each is so similar.
Sugar Alcohols (Polyols, Sugar Replacers)
Sugar(s) can provide a lot of calories and contribute to tooth decay. Thus there are many other compounds that are used as alternatives to sugar that have been developed or discovered. We will first consider sugar alcohols and then the alternative sweeteners in subsequent sections.
Below you can see the structure of three common sugar alcohols: xylitol, sorbitol, and mannitol.
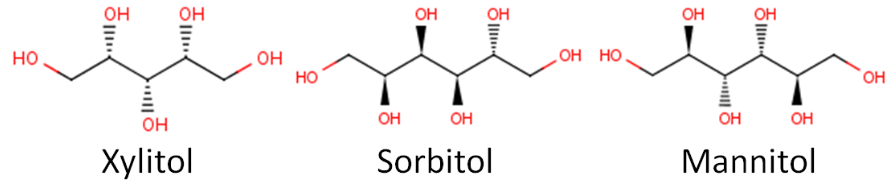
Remember that hydroxyl groups are (\(\ce{-OH}\)), and you can see many of them in these structures. An alcohol is a carbon containing compound that includes at least one hydroxyl group.
Sugar alcohols are also known as "sugar replacers", because some in the public might get confused by the name sugar alcohol. Some might think a sugar alcohol is a sweet alcoholic beverage. Another name for them is nutritive sweeteners, which indicates that they do provide calories. Sugar alcohols are less sweet and provide about half the calories of sucrose as shown below. The name polyols also seems to be increasingly used to describe these compounds. It should be noted that consuming a large amount of sugar alcohols has a laxative effect.
| Sweetener | Relative Sweetness | Energy (kcal/g) |
|---|---|---|
| Lactose | 0.2 | 4* |
| Maltose | 0.4 | 4 |
| Glucose | 0.7 | 4 |
| Sucrose | 1.0 | 4 |
| Fructose | 1.2-1.8 | 4 |
| Erythritol | 0.7 | 0.4 |
| Isomalt | 0.5 | 2.0 |
| Lactitol | 0.4 | 2.0 |
*Differs based on a person’s lactase activity
Sugars are fermented by bacteria on the surfaces of teeth. Acid is a product of this fermentation, which results in decreased pH (higher acidity) that leads to tooth decay and, potentially, cavity formation. The major advantage of sugar alcohols over sugars is that sugar alcohols are not fermented by bacteria on the tooth surface. There is a nice picture of this process in the link below as well as a video explaining the process of cavity development.
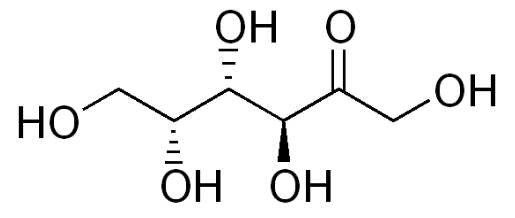
Tagatose
While not a sugar alcohol, tagatose is very similar to sugar alcohols. Tagatose is an isomer of fructose, that provides a small amount of energy (1.5 kcal/g). 80% of tagatose reaches the large intestine, where it is fermented by bacteria, meaning it has a prebiotic-type effect6. Notice the similarity in structure of tagatose to sugar alcohols, the only difference being a ketone (\(\ce{=O}\)) instead of an alcohol (\(\ce{-OH}\)) group.
Alternative Sweeteners
Alternative sweeteners are simply alternatives to sucrose and other mono- and disaccharides that provide sweetness. Many have been developed to provide zero-calorie or low calorie sweetening for foods and drinks.
Because many of these provide little to no calories, these sweeteners are also referred to as non-nutritive sweeteners (FDA is using high-intensity sweeteners to describe these products10). Aside from tagatose (described in the sugar alcohol section), all sweeteners on the list below meet this criteria. Aspartame does provide calories, but because it is far sweeter than sugar, the small amount used does not contribute meaningful calories to a person's diet. Until the FDA allowed the use of stevia, this collection of sweeteners were commonly referred to as artificial sweeteners because they were synthetically or artificially produced. Since then other similar "natural" sweeteners have also been allowed to be used. The link below summarizes the characteristics of the FDA approved high-intensity sweeteners.
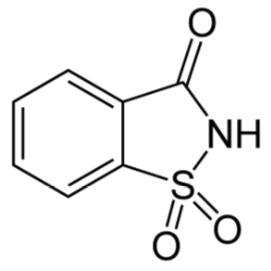
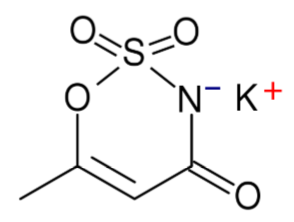
Saccharin
Saccharin is the oldest of the artificial sweeteners. However, it should be noted that both sweet and bitter taste receptors are triggered by it, so for some people it has an aftertaste that is off-putting11,12. It has been found that this bitter or metallic flavor can sometimes be masked by mixing alternative sweeteners13. It is a heat-stable alternative sweetener.
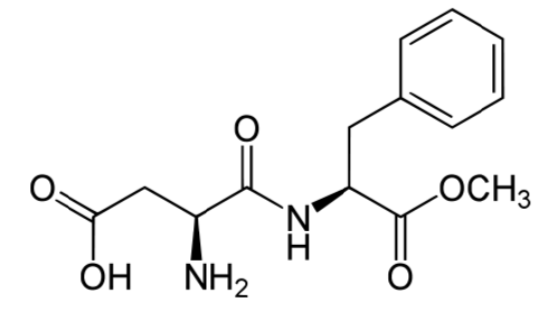
Aspartame
Aspartame is made up of 2 amino acids (phenylalanine and aspartate) and a methyl (\(\ce{-CH3}\)) group. The compound is broken down during digestion into the individual amino acids. This is why it provides 4 kcal/g, just like protein11. However, it is still considered noncaloric because it is so sweet that we use very small amounts that don’t provide any meaningful caloric value. Because it can be broken down to phenylalanine, products that contain aspartame contain the following message: "Phenylketonurics: Contains phenylalanine." Phenylketonuria (PKU) will be covered in greater detail in the protein section. When heated, aspartame breaks down and loses its sweet flavor7.
Neotame
Neotame is like aspartame version 2.0. Neotame is structurally identical to aspartame except that it contains an additional side group (bottom of the figure below, which is flipped backwards to make it easier to compare their structures). While this looks like a minor difference, it has profound effects on the properties of neotame. Neotame is much sweeter than aspartame and is heat-stable. It can still be broken down to phenylalanine, but such small amounts are used that it is not a concern for those with PKU7,11.
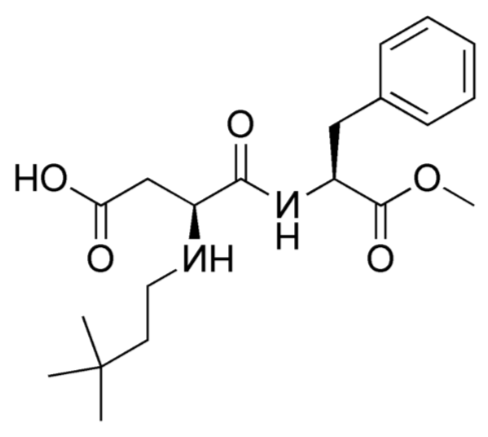
Advantame
The sweetest alternative sweetener approved by the FDA in 2014 is advantame. It is heat-stable and does not have a trade name yet10. Notice it also has a similar structure to aspartame and neotame. Like Neotame, it can be broken down to phenylalanine, but such small amounts are used that it is not a concern for those with PKU. However, it has a much higher acceptable daily intake than Neotame11, meaning there is less concern about adverse effects from consuming too much.
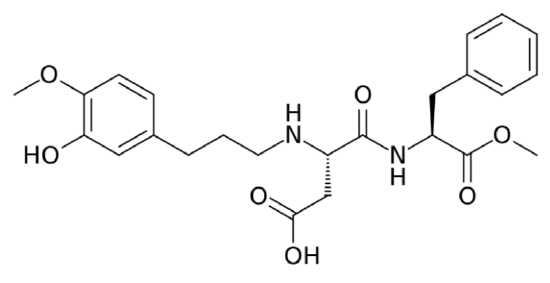
Acesulfame-Potassium (K)
Acesulfame-potassium (\(\ce{K}\)) is not digested or absorbed, therefore it provides no energy or potassium to the body7. It is a heat-stable alternative sweetener.
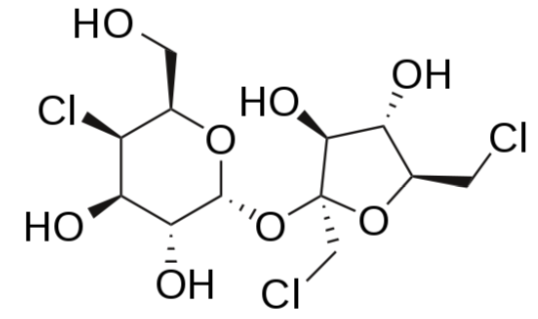
Sucralose
Sucralose is structurally identical to sucrose except that 3 of the hydroxyl groups (\(\ce{OH}\)) are replaced by chlorine molecules (\(\ce{Cl}\)). This small change causes sucralose to not be digested and as such is excreted in feces7,11. It is a heat-stable alternative sweetener.
Stevia
Stevia is derived from a South American shrub, with the leaves being the sweet part. The components responsible for this sweet taste are a group of compounds known as steviol glycosides. The structure of steviol is shown below.
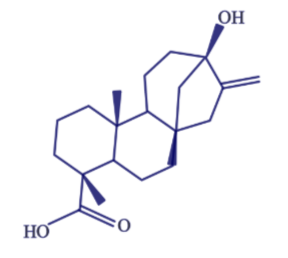
The term glycoside means that there are sugar molecules bonded to steviol. The two predominant steviol glycosides are stevioside and rebaudioside A. The structure of these two steviol glycosides is very similar21. The structure of stevioside is shown below as an example.
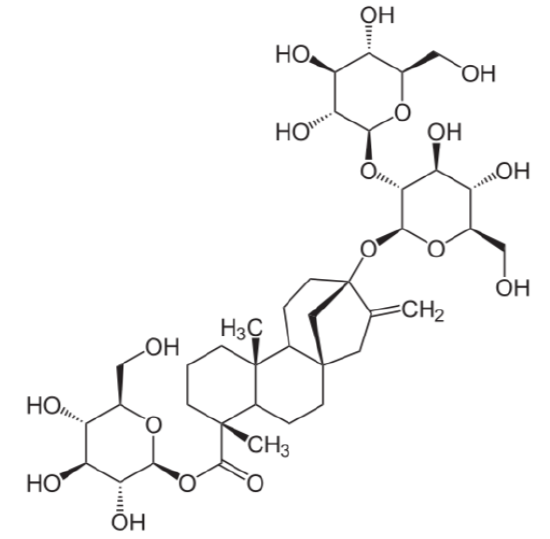
The common name for a sweetener containing primarily rebaudioside A is rebiana. Stevia sweeteners are heat-stable and have been marketed as a natural alternative sweeteners, something that has been challenged by lawsuits as described in the following link.
Luo Han Guo Fruit Extracts
Luo Han Guo (aka Siraitia grosvenrii Swingle, monk) fruit extracts are a newer, natural heat-stable alternative sweetener option derived from a native Chinese fruit. These extracts are sweet because of the mogrosides they contain10. The structure of a mogroside is shown below.
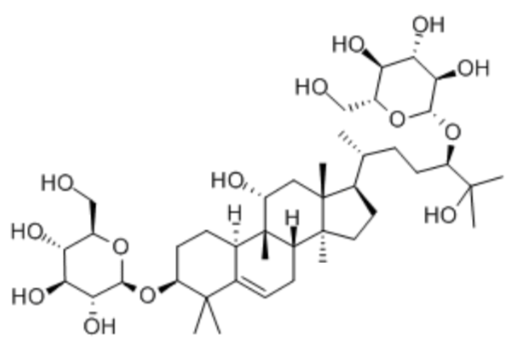
Figure \(\PageIndex{15}\): Structure of a mogroside23
Sweet Proteins
These alternative sweetener proteins work by activating the same taste receptors as sucrose.
Thaumatin
Thaumatin is another natural alternative sweetener protein derived from the fruit of West African katemife plant, what is unique about it is that is an intensely sweet (2000-3000X sweet as sucrose) protein10,24. It does lose its sweetness during heating.

Figure \(\PageIndex{15}\): Structure of a thaumatin25
Brazzein
Brazzein is another natural alternative sweetener protein from the West African oubli fruit. It is 500-2000 times as sweet as sucrose and is heat-stable24.

Figure \(\PageIndex{15}\): Structure of a brazzein23
References
- http://www.fda.gov/food/ingredientsp.../ucm324856.htm
- www.foodnavigator-usa.com/Mar...eeteners-drops
- https://pubchem.ncbi.nlm.nih.gov/com...ol#section=Top
- https://pubchem.ncbi.nlm.nih.gov/com...ol#section=Top
- https://pubchem.ncbi.nlm.nih.gov/com...ol#section=Top
- Wardlaw GM, Hampl J. (2006) Perspectives in nutrition. New York, NY: McGraw-Hill.
- Whitney E, Rolfes SR. (2008) Understanding nutrition. Belmont, CA: Thomson Wadsworth.
- http://en.Wikipedia.org/wiki/File:Tagatose.png
- www.fda.gov/AboutFDA/Transpar.../ucm214865.htm
- http://www.fda.gov/food/ingredientsp.../ucm397725.htm
- Byrd-Bredbenner C, Moe G, Beshgetoor D, Berning J. (2009) Wardlaw's perspectives in nutrition. New York, NY: McGraw-Hill.
- Fernstrom JD, Munger SD, Sclafani A, de Araujo IE, Roberts A, Molinary S (2012) Mechanisms for Sweetness. J Nutr 142 (6) 1134S–1141S.
- Behrens M, Blank K, Meyerhof W. (2017) Blends of Non-caloric Sweeteners Saccharin and Cyclamate Show Reduced Off-Taste due to TAS2R Bitter Receptor Inhibition. Cell Chem Biol 24 (10) 1199-1204.
- https://en.Wikipedia.org/wiki/Saccha...:Saccharin.svg
- en.Wikipedia.org/wiki/Aspartame
- en.Wikipedia.org/wiki/File:Neotame.png
- en.Wikipedia.org/wiki/File:Advantame.svg
- en.Wikipedia.org/wiki/File:AcesulfameK.svg
- en.Wikipedia.org/wiki/File:Sucralose2.svg
- en.Wikipedia.org/wiki/File:Steviol.svg
- Carakostas MC, Curry LL, Boileau AC, Brusick DJ. (2008) Overview: The history, technical function and safety of rebaudioside A, a naturally occurring steviol glycoside, for use in food and beverages. Food and Chemical Toxicology 46 Suppl 7: S1.
- en.Wikipedia.org/wiki/File:Steviosid.svg
- en.Wikipedia.org/wiki/File:Mogroside_II_E.gif
- https://oobli.com/blogs/news/what-are-sweet-proteins
- https://en.wikipedia.org/wiki/Thauma...tin_I_1RQW.png
- https://en.wikipedia.org/wiki/Brazze...Brazzeinmj.png


