2.6: Lipids - Fatty Acid Naming, Food Sources, Essential Fatty Acids and Eicosanoids
- Page ID
- 41718
Fatty Acid Naming & Food Sources
There are three naming systems used for fatty acids:
- Delta nomenclature
- Omega nomenclature
- Common names
The omega nomenclature and common names are used more in the field of nutrition than the delta nomenclature when describing specific fatty acids.
Delta Nomenclature
For delta nomenclature you need to know 3 things:
- Number of carbons in the fatty acid
- Number of double bonds
- Number of carbons from the carboxylic acid (alpha) end to the first carbon in the double bond(s)
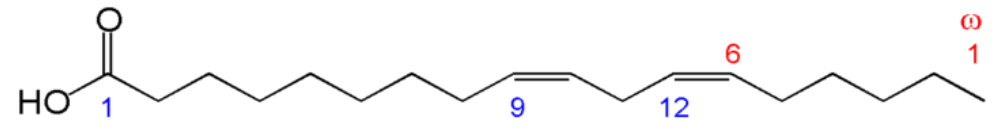
Let's consider the example in the figure below.
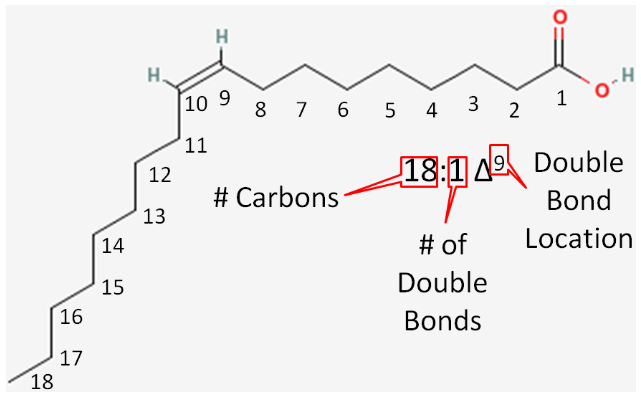
- Number of carbons in the fatty acid = 18
- Number of double bonds = 1
- Number of carbons from the carboxylic acid end to the first carbon in the double bond = 9
This is then written as shown in Figure \(\PageIndex{10}\).
Omega Nomenclature
The omega nomenclature is almost exactly the same as the delta nomenclature, the only differences being:
- Carbons are counted from the methyl (omega) end instead of the carboxylic acid end
- The omega symbol is used instead of the delta symbol
For omega nomenclature you need to know 3 things:
- Number of carbons in the fatty acid
- Number of double bonds
- Number of carbons from the methyl end (aka Omega end) to the first carbon in the double bond closest to the methyl end
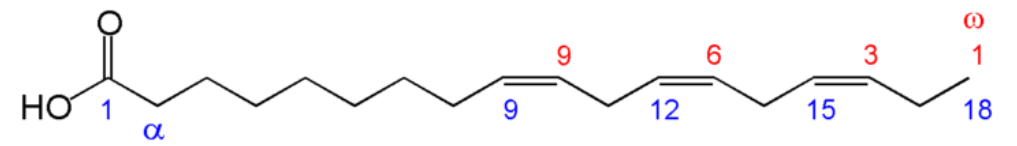
We will again consider the same fatty acid.
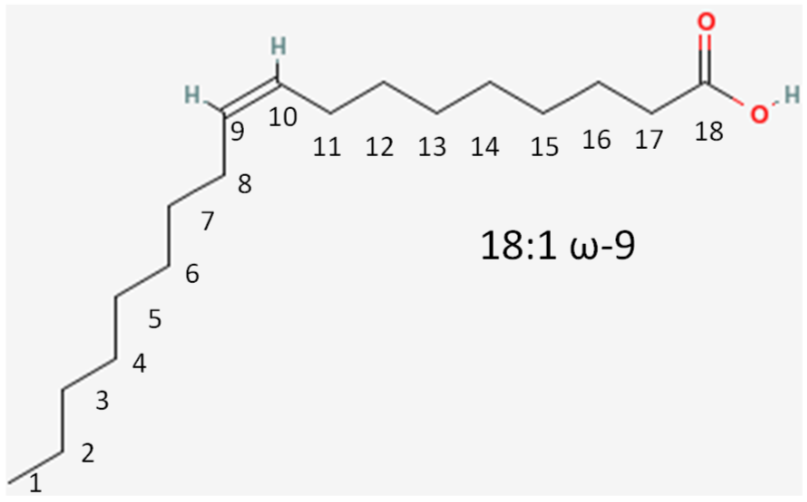
- Number of carbons in the fatty acid = 18
- Number of double bonds = 1
- Number of carbons from the methyl (aka omega) end to the first carbon in the double bond closest to the methyl end = 9
If it is a saturated fatty acid, then the omega nomenclature is not added to the end of the name. If it is an 18 carbon saturated fatty acid, then it would be named 18:0.
This is written as shown in Figure \(\PageIndex{11}\). Instead of an omega prefix, the prefix n- (i.e. n-3) is also commonly used.
Common Names
The common names of fatty acids are something that, for the most part, have to be learned/memorized. The common name of the fatty acid we have been naming in this section is oleic acid.
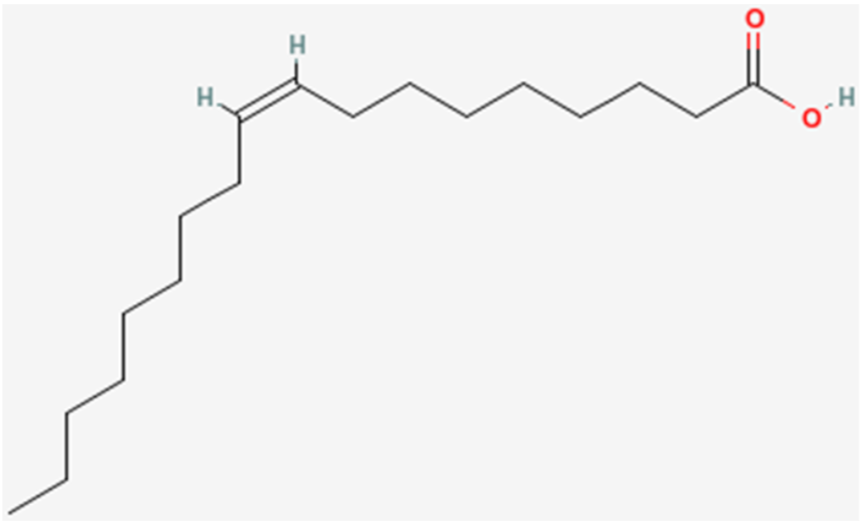
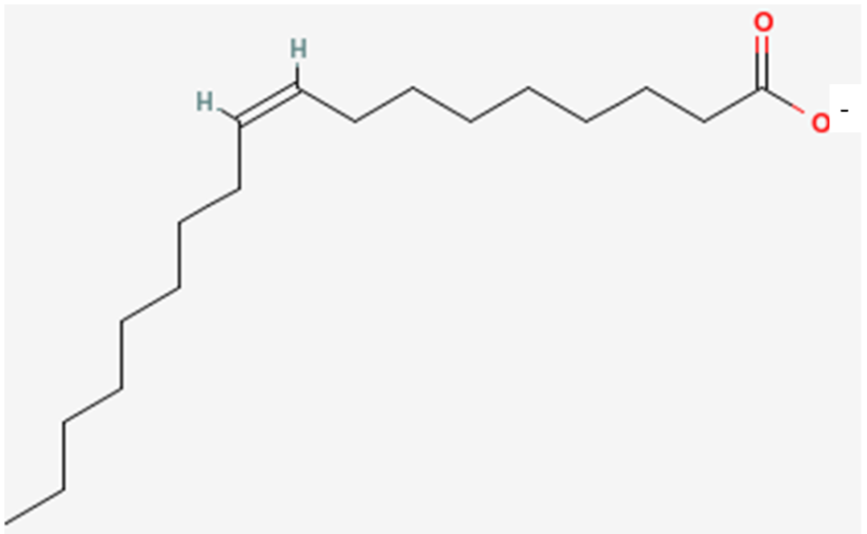
However, it can also be called oleate. The only difference is that, instead of a carboxylic acid on the end of the fatty acid, it has been ionized to form a salt (shown below). This is what the -ate ending indicates and the two names are used interchangeably. This is true for all fatty acids.
The table below gives the common names and food sources of some common fatty acids.
| Omega Name | Common Name |
|---|---|
| 4:0 | Butyric Acid |
| 12:0 | Lauric Acid |
| 14:0 | Myristic Acid |
| 16:0 | Palmitic acid |
| 18:0 | Stearic Acid |
| 20:0 | Arachidic Acid |
| 24:0 | Lignoceric Acid |
| 18:1 (n-9) | Oleic Acid |
| 18:2 (n-6) | Linoleic Acid |
| 18:3 (n-3) | Alpha-linolenic Acid |
| 20:4 (n-6) | Arachidonic Acid |
| 20:5 (n-3) | Eicosapentanoic Acid |
| 22:6 (n-3) | Docosahexanoic Acid |
Food Sources of Fatty Acids
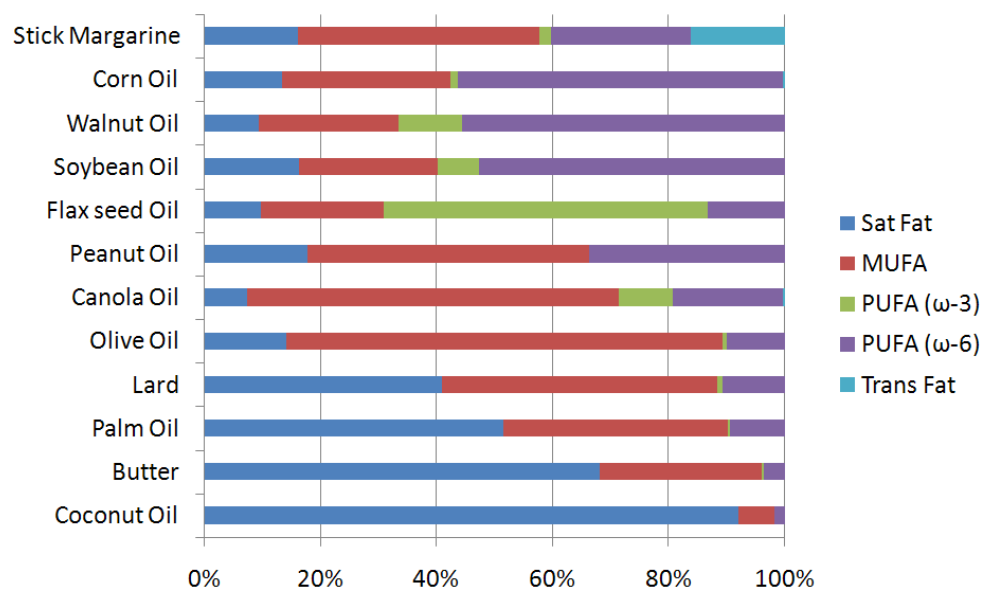
After going through this wide array of fatty acids, you may be wondering where they are found in nature. The figure below shows the fatty acid composition of certain oils and oil-based foods. As you can see, most foods contain a mixture of fatty acids. Stick margarine is the only product in the figure that contains an appreciable amount of trans fatty acids. Corn, walnut, and soybean are rich sources of n-6 polyunsaturated fatty acids, while flax seed is fairly unique among plants in that it is a good source of n-3 polyunsaturated fatty acids. Canola and olive oil are rich sources of monounsaturated fatty acids. Lard, palm oil, butter and coconut oil all contain a significant amount of saturated fatty acids.
Essential Fatty Acids & Eicosanoids
The two essential fatty acids are:
- linoleic acid (omega-6)
- alpha-linolenic (omega-3)
These fatty acids are essential because we can not synthesize them. This is because we do not have an enzyme capable of adding a double bond (desaturating) at the omega-6 and 3 positions. We do have an enzyme that can add the omega-9 bond, which is why it is not essential. The structures of the two essential fatty acids are shown below.
However, we do possess enzymes that can take the essential fatty acids, elongate them (add two carbons to them), and then further desaturate them (add double bonds) to other omega-6 and omega-3 fatty acids. Thus, there are 2 families of fatty acids that the majority of polyunsaturated fatty acids fit into as shown below.
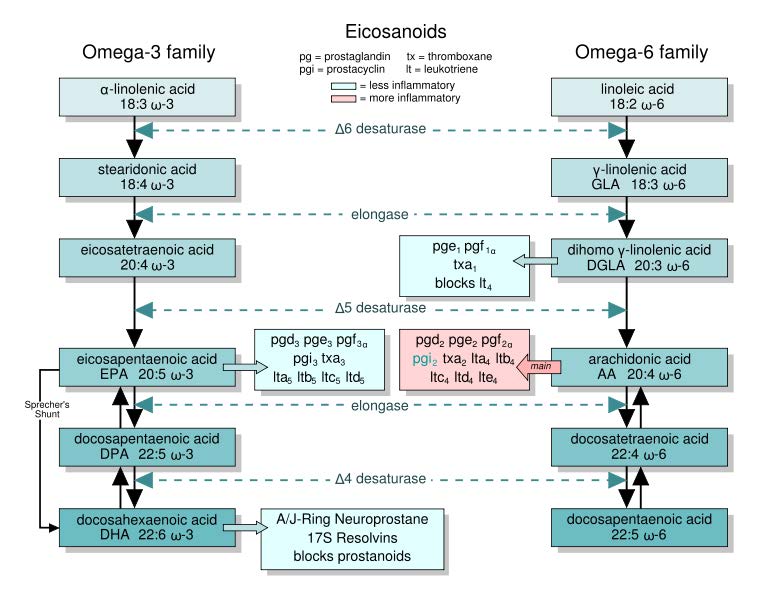
The same enzymes are used for both omega-6 and omega-3 fatty acids. However, we cannot convert omega-3 fatty acids to omega-6 fatty acids or omega-6 fatty acids to omega-3 fatty acids. Among these families, the omega-3 fatty acid, eicosapentaenoic acid, and the omega-6 fatty acids, dihomo gamma-linolenic acid and arachidonic acid, are used to form compounds known as eicosanoids. These 20 carbon fatty acid derivatives are biologically active in the body (like hormones, but they act locally in the tissue they are produced). There are four classes of eicosanoids:
- Prostaglandins (PG)
- Prostacyclins (PC)
- Thromboxanes (TX)
- Leukotrienes (LT)
Some examples of eicosanoid structures are shown in the figure below:

The difference in the effects and outcomes of omega-6 and omega-3 fatty acid intake is primarily a result of the eicosanoids produced from them. Omega-6 fatty acid derived eicosanoids are more inflammatory than omega-3 fatty acid derived eicosanoids. As a result, omega-3 fatty acids are considered anti-inflammatory because replacing the more inflammatory omega-6 fatty acid derived eicosanoids with omega-3 fatty acid derived eicosanoids will decrease inflammation. As an example of the action of eicosanoids, aspirin works by inhibiting the enzymes cyclooxygenase 1 (Cox-1) and cyclooxygenase 2 (Cox-2). These enzymes convert arachidonic acid into inflammatory prostaglandins as shown below.
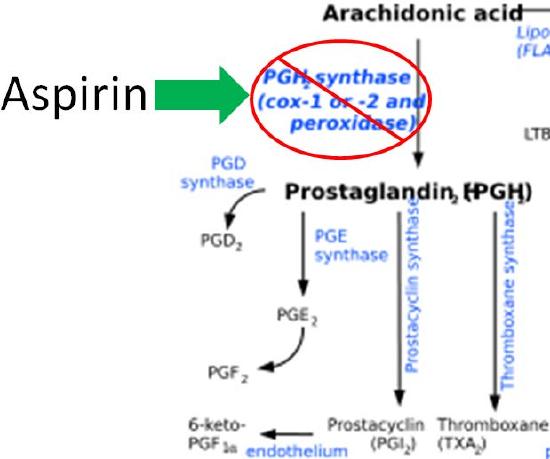
You have probably heard that you should get more omega-3s in your diet, and in general polyunsaturated fatty acids are considered healthy. However, since omega-3 fatty acids are competing for the same enzymes as omega-6 fatty acids, and because the omega-6 fatty acids are more inflammatory, consuming too many omega-6s is probably more detrimental than helpful. As a result, there was interest in the dietary omega-3:omega-6 fatty acid ratio. For most Americans, the ratio was believed to be too high, at almost 10-20 times more omega-6 fatty acids than omega-3 fatty acids12. More recently the Omega-3 index, which is the EPA and DHA red blood cell (erythrocyte) content, has gained greater attention as a better indicator17. The table below shows good food sources of some selected omega-3 and omega-6 fatty acids.
| Fatty Acid | Good Food Sources |
|---|---|
| Linoleic Acid (LA, n-6) | Safflower Oil, Corn Oil, Sunflower Oil |
| Arachidonic Acid (AA, n-6) | Eggs, Meat |
| Alpha-Linolenic Acid (ALA, n-3) | Walnuts, Flaxseed (linseed), Canola (rapeseed), and Soybean Oils |
| Eicosapentaenoic Acid (EPA, n-3) | Fatty Fish & Fish Oils |
| Docosahexanoic Acid (DHA, n-3) | Fatty Fish & Fish Oils |
Even though Figure \(\PageIndex{17}\) illustrates the conversion of alpha-linolenic acid to eicosapentanoic acid and docosahexanoic acid, this conversion is actually quite limited. Only 0.2-8% and 0-4% of alpha-linolenic acid is converted to eicosapentanoic acid and docosahexanoic acid, respectively14. Thus, dietary consumption is the most effective way to get the longer chain fatty acids (eicosapentanoic acid and docosahexanoic acid) in our bodies. It is less clear whether alpha-linolenic acid consumption is as beneficial as eicosapentanoic acid and docosahexanoic acid) but a study found it to be equally effective in decreasing blood triglyceride concentrations. In that study, DHA had the added positive benefit of increasing high-density lipoprotein15. These are all positive outcomes that are expected to reduce the risk of developing cardiovascular disease. However, there is debate about the effectiveness of fish oil, most of the recommendations are focusing on an EPA supplement, if they think there should be any supplement, like described in the following 2 articles.
Essential Fatty Acid Deficiency
Essential fatty acid deficiency is rare and unlikely to occur, but the symptoms are:
- Growth inhibition
- Reproductive problems
- Skin lesions
- Neurological and visual problems
References
- Gropper SS, Smith JL, Groff JL. (2008) Advanced nutrition and human metabolism. Belmont, CA: Wadsworth Publishing.
- www.nutritiondata.com
- en.Wikipedia.org/wiki/File:LAnumbering.png
- en.Wikipedia.org/wiki/File:ALAnumbering.png
- en.Wikipedia.org/wiki/File:EF...icosanoids.svg
- en.Wikipedia.org/wiki/File:Pr...glandin_E1.svg
- en.Wikipedia.org/wiki/File:Thromboxane_A2.png
- en.Wikipedia.org/wiki/File:Leukotriene_B4.svg
- en.Wikipedia.org/wiki/File:Pr...glandin_I2.png
- en.Wikipedia.org/wiki/File:Leukotriene_E4.svg
- en.Wikipedia.org/wiki/File:Ei..._synthesis.svg
- Simopoulos AP. (2008) The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med 233(6): 674.
- Harris. WS (2018) The Omega-6:Omega-3 ratio: A critical appraisal and possible successor. Prostaglandins Leukot Essent Fatty Acids 132: 34-40.
- Arterburn LM, Hall EB, Oken, H. (2006) Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr 83(suppl) 1467.
- Egert S, Kannenberg F, Somoza V, Erbersdobler H, Wahrburg U. (2009) Dietary alpha-linolenic acid, EPA, and DHA have differential effects on LDL fatty acid composition but similar effects on serum lipid profiles in normolipidemic humans. J Nutr 139(5): 861.


