9.2: Vitamin E
- Page ID
- 21018
There are 8 different forms of vitamin E: 4 tocopherols and 4 tocotrienols. The difference between tocopherols and tocotrienols is that the former have a saturated tail, while the latter have an unsaturated tail. Within tocopherols and tocotrienols, the difference between the different forms is the position of the methyl groups on the ring. The 4 different forms within the tocopherol and tocotrienols are designated by the Greek letters: alpha, beta, gamma, and delta. The difference in these structures is shown in the figures below.
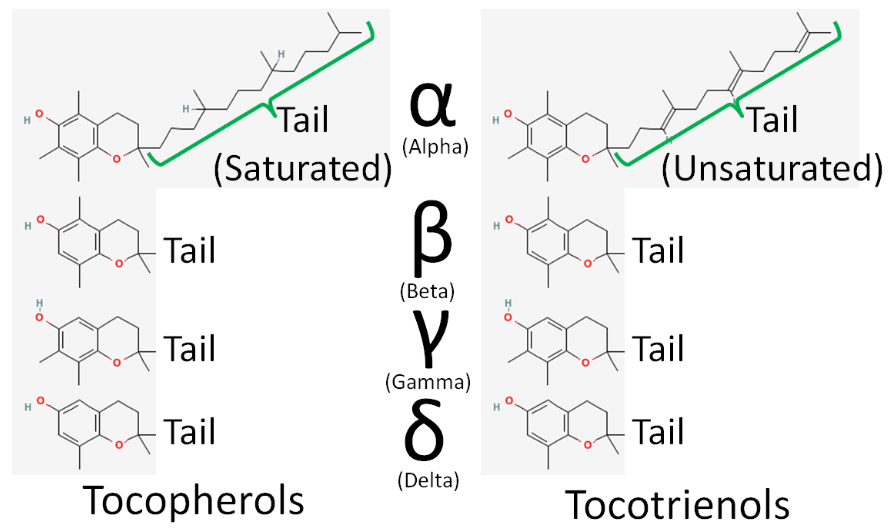
For reasons that you will learn about later in this subsection, the primary form of vitamin E found in the body is alpha-tocopherol. The major, and possibly only, function of vitamin E is as an antioxidant. When it serves as an antioxidant it forms an alpha-tocopherol radical, as shown below.

Alpha-tocopherol is believed to be the first part of an antioxidant network (shown below) where it is oxidized to donate an electron to stabilize reactive oxygen species. Alpha-tocopherol radical can then be reduced by the donation of an electron from ascorbate.
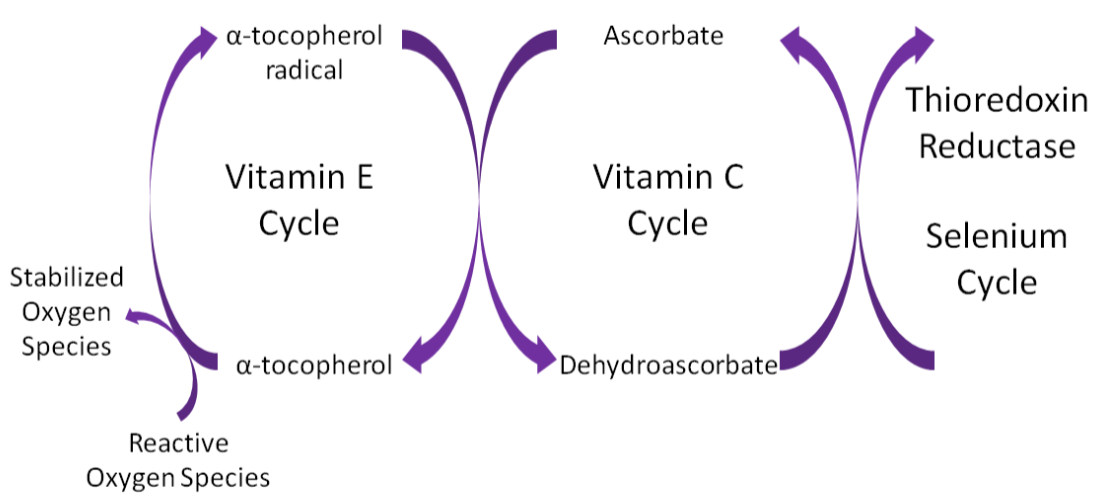
To help protect the antioxidant function of alpha-tocopherol (by preventing the formation of alpha-tocopherol radical) in foods and during digestion, some manufacturers have added compounds to this site of alpha-tocopherol through ester bonds. These are referred to as alpha-tocopherol derivatives or alpha-tocopherol esters. The most common forms are alpha-tocopherol acetate, alpha-tocopherol succinate, and alpha-tocopherol phosphate (Ester-E®). The figures below show the structure of alpha-tocopherol acetate, and the structure of succinic acid.
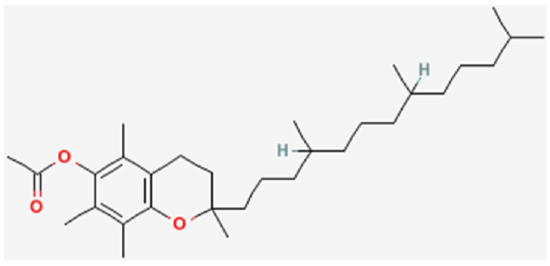
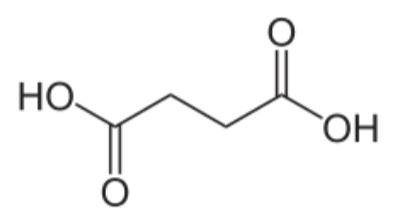
Alpha-tocopherol derivatives, such as acetate in alpha-tocopherol acetate, are cleaved prior to absorption in the small intestine by esterases, meaning that alpha-tocopherol is absorbed, not the alpha-tocopherol derivative.
Alpha-Tocopherol: Natural vs. Synthetic
In addition to being found naturally in foods, alpha-tocopherol can also be synthesized. It is important to know whether alpha-tocopherol is natural or synthetic because the stereochemistry (spatial arrangement) differs between these forms. In some cases stereochemistry is used to three dimensionally depict whether a molecule is coming out towards you or alternatively away from you. Alpha-tocopherol contains 3 chiral centers (non-superimposable mirror images) designated as R or S.
The 3 chiral centers in alpha-tocopherol are located at the 2, 4’, and 8’ positions. You can see the full numbering of tocopherols in the link below. In short, the rings are normal numbers and the tail are prime numbers.
The figure below shows the 3 chiral centers without the other numbers.
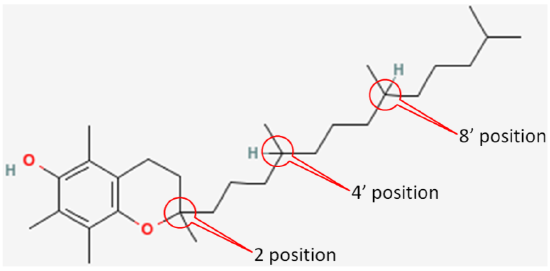
In natural alpha-tocopherol, all 3 chiral centers are in the R configuration. Thus, it is designated RRR-alpha-tocopherol. The R’s represent the 2, 4’, and 8’ positions of alpha-tocopherol, respectively, as shown below4.
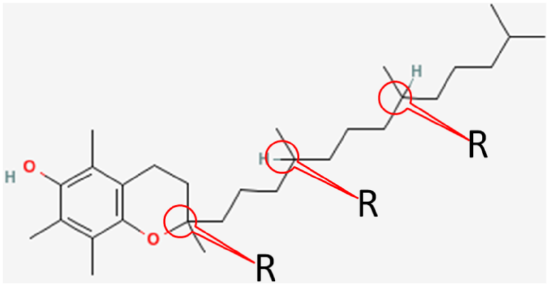
Synthetic alpha-tocopherol is a racemic (equal) mixture of all the different possibilities at the three chiral centers. These are:
RRR
RRS
RSS
RSR
SRR
SSR
SSS
SRS
The two forms of alpha-tocopherol are designated (these are placed before alpha-tocopherol to indicate whether it is natural or synthetic) as listed below:
- Natural
New designation: RRR-alpha-tocopherol (because all 3 positions are RRR)
Old designation: d-alpha-tocopherol
- Synthetic
New designation: all-rac-alpha-tocopherol (because it is a racemic mixture)
Old designation: dl-alpha-tocopherol
The old d and dl designations were describing the chemical structure that are sometimes still used. Keep in mind the natural and synthetic are describing the stereochemistry of alpha-tocopherol and not whether it is naturally derived. For example, there are natural alpha-tocopherol derivatives where the derivatives are added through synthetic procedures.
Vitamin E Absorption, Metabolism, & Excretion
You might be saying to yourself, “who cares about natural versus synthetic alpha-tocopherol.” But the small change in stereochemistry makes a big difference in how alpha-tocopherol is maintained in the body.
All forms of vitamin E (tocopherols, tocotrienols) are absorbed equally. Fat-soluble vitamins are handled like lipids and thus are incorporated into chylomicrons that have triglycerides removed by lipoprotein lipase. The chylomicron remnants containing the different forms of vitamin E are then taken up by the liver. The figure below shows the absorption, metabolism, and excretion of vitamin E.
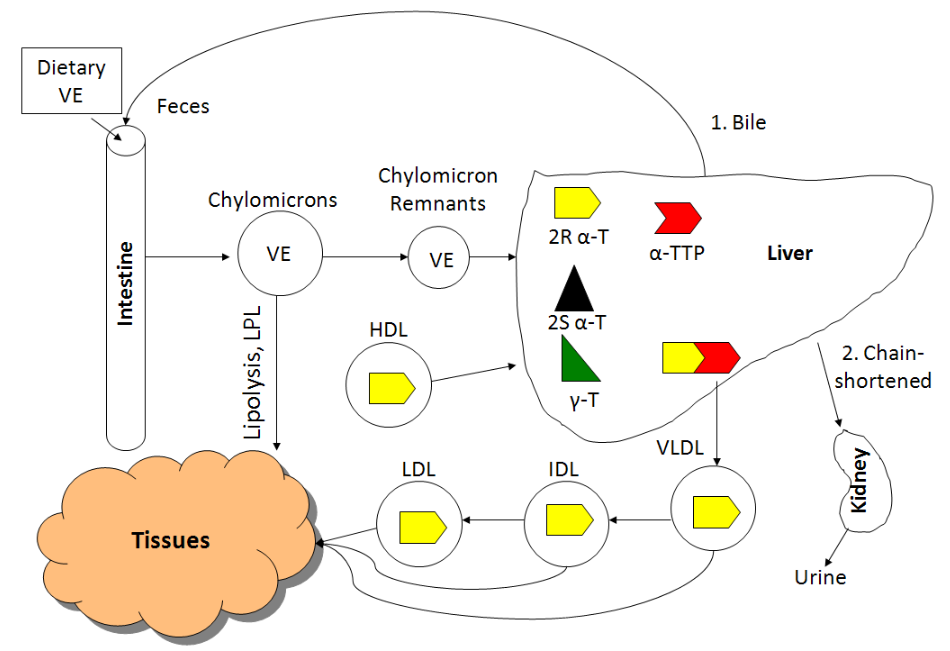
The liver contains a protein called alpha-tocopherol transfer protein (alpha-TTP), which is responsible for maintaining higher levels of alpha-tocopherol in the body. Alpha-TTP preferentially binds to 2R alpha-tocopherol and helps facilitate its incorporation into VLDL. 2R means any form of alpha-tocopherol in which the 2 position is in the R conformation. The following table summarizes the forms of alpha-tocopherol that bind well to alpha-TTP, and those that don't bind well to alpha-TTP.
| Do not bind well to alpha-TTP | Bind well to alpha-TTP |
|---|---|
| SRR | RRR |
| SSR | RRS |
| SSS | RSS |
| SRS | RSR |
Other forms of vitamin E (gamma-tocopherol, tocotrienols) also don't bind well to alpha-TTP and thus, are found in lower levels than alpha-tocopherol in the body. The following graph shows plasma (liquid component of blood) vitamin E levels from a study in which subjects were given 150 mg each of RRR-alpha-tocopherol, all-rac-alpha-tocopherol, or gamma-tocopherol5.
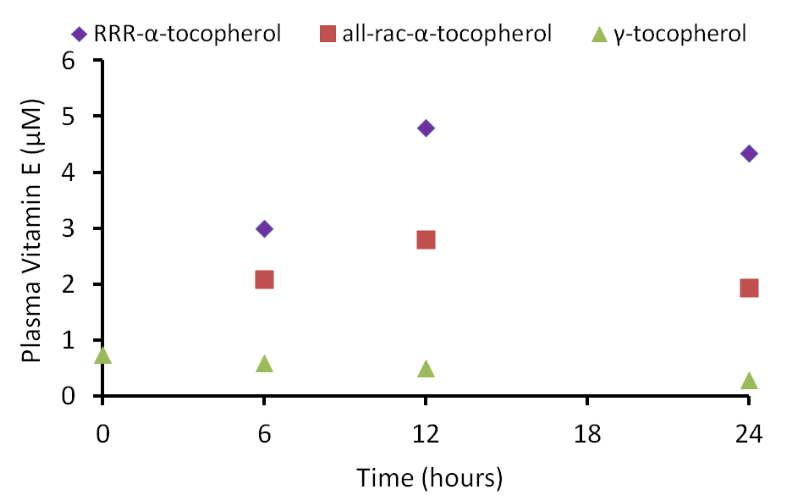
As you can see in the figure, there was a greater rise in the plasma alpha-tocopherol levels after receiving RRR-alpha-tocopherol vs. all-rac-alpha-tocopherol. This is not a surprise because approximately 50% of all-rac-alpha-tocopherol is 2R alpha-tocopherol that binds well with alpha-TTP. You can also see that the plasma gamma-tocopherol concentration is much lower than either natural or synthetic alpha-tocopherol.
From VLDL and subsequent lipoproteins, vitamin E reaches tissues, with most vitamin E in the body being found in the adipose tissue. There are 2 main routes of vitamin E excretion. The major route of excretion is through bile that is then excreted in feces. The second route is in the urine after vitamin E is chain-shortened in a process similar to beta-oxidation to make them more water-soluble.
Dietary Vitamin E & Amounts Found in Body
The best food sources of vitamin E are primarily oils and nuts. As you can see below, the forms of vitamin E that nuts and oils contain varies, with the two major forms being alpha and gamma-tocopherol. Soybean, corn, and flaxseed oils are good sources of gamma-tocopherol. Palm and canola oils contain almost equal amounts of alpha-tocopherol and gamma-tocopherol. Safflower oil, almonds, sunflower oil, and wheat germ oil are good sources of alpha-tocopherol. Beta-tocopherol and delta-tocopherol are found in lower levels in foods. Tocotrienols, for the most part, are not found in high levels in the diet. The amount of tocopherols in different nuts and oils are shown in the figure below.
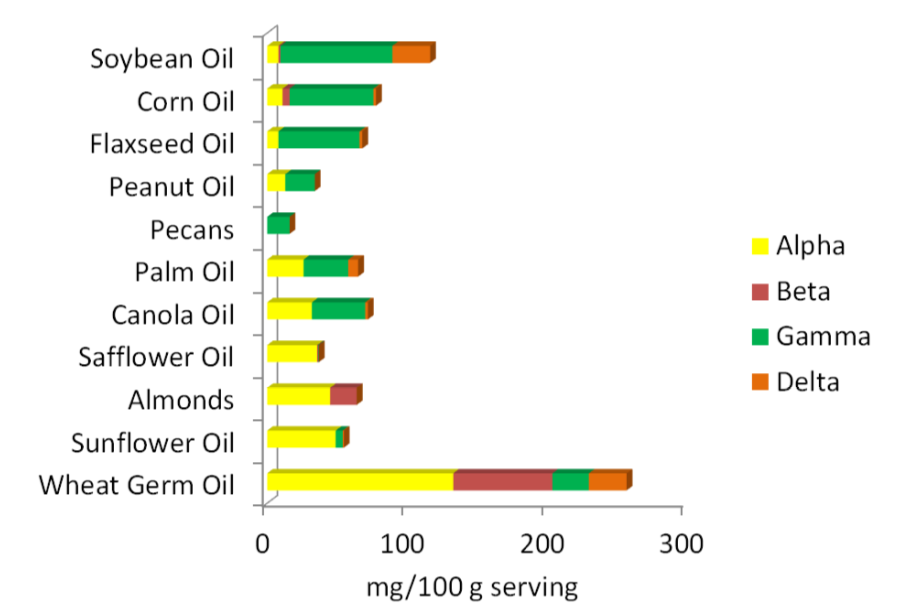
Three-fourths of the oil Americans consume is soybean oil. As a result, it is estimated that we consume 2-4 times more gamma-tocopherol than alpha-tocopherol. Europeans consume more olive, sunflower, and canola oil and thus are believed to consume at least 2 times more alpha-tocopherol than gamma-tocopherol6.
Despite Americans’ higher intake of gamma tocopherol compared to other countries, our serum (liquid portion of blood without coagulation proteins) concentrations do not differ much as illustrated in the table below.
| Location | Gamma-tocopherol | Alpha-tocopherol |
|---|---|---|
| USA 1* | 2-7 | 15-20 |
| USA 2 | 5.4 | 22.3 |
| USA 3 | 2.5 | 21.8 |
| Costa Rica | 2.7 | 28.6-31.8 |
| France | 1.05-1.28 | 26.7 |
| Ireland | 1.74-1.87 | 26.3 |
| The Netherlands | 2.3 | 23.9-25.5 |
| Spain | 0.88-1.14 | 27.4-28.3 |
| Italy | 1.29 | 24.3 |
| Sweden | 3.2 | 23.8 |
| Lithuania | 1.64 | 21.7 |
| Austria | 1.48 | 21.1 |
Tissue concentrations, for the most part, also indicate a greater accumulation of alpha-tocopherol than gamma-tocopherol as shown in the table below.
| Tissue | Gamma-tocopherol | Alpha-tocopherol |
|---|---|---|
| Skin | 180 | 127 |
| Adipose | 176 | 440 |
| Muscle | 107 | 155 |
Vitamin E Deficiency & Toxicity
Vitamin E deficiency is extremely rare. Depletion studies require years on a vitamin E-deficient diet to cause deficiency7. Deficiency primarily occurs in people with lipid malabsorption problems or Ataxia with Isolated Vitamin E Deficiency (AVED). Individuals with AVED have a mutation in their alpha-TTP that prevents it from functioning correctly. The primary symptoms of vitamin E deficiency are neurological problems.
High levels of vitamin E intake do not result in a noted toxicity. However, higher levels of intake of alpha-tocopherol (like achieved by taking supplements) are associated with decreased blood coagulation. In particular, hemorrhagic stroke has been linked to high alpha-tocopherol intake levels. The link below shows that in this condition a blood vessel ruptures or leaks in the brain.
It is believed that this increased bleeding risk is due to an alpha-tocopherol metabolite that has anti-vitamin K activity. This potential antagonism will be described more in the vitamin K section.
Vitamin E DRI & IUs
Before 2001, all forms of vitamin E contributed to the RDA, using a measure called alpha-tocopherol equivalents. This indicator essentially provided a value for all forms relative to alpha-tocopherol. In 2001, the Dietary Reference Intake (DRI) committee decided only 2R forms of alpha-tocopherol should be used to estimate the requirement, because these forms bind to alpha-TTP. Thus, other forms of vitamin E (gamma-tocopherol, tocotrienols etc.) do not count toward the requirement and the unit is now mg of alpha-tocopherol. As a result, soybean, corn, and flaxseed oils, which are good sources of gamma-tocopherol, are no longer considered to be good sources of vitamin E. The figure below is a reminder of the tocopherol content of different nuts and oils.
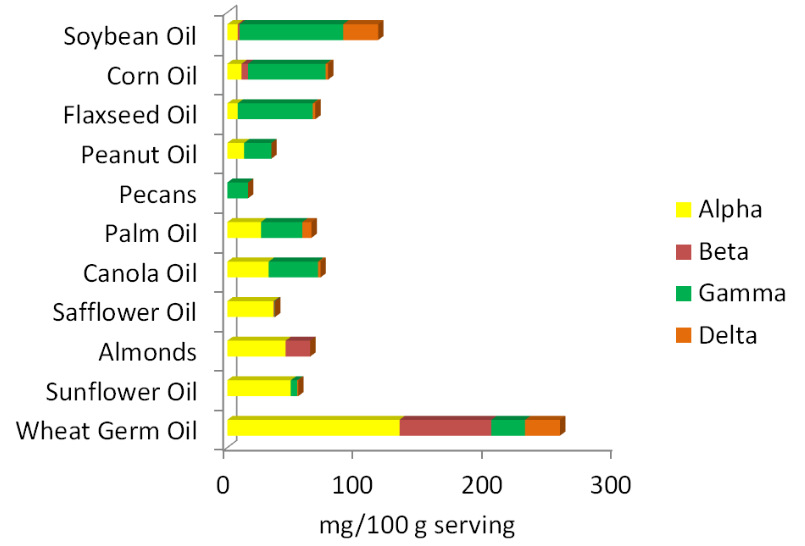
Another level of complexity is added by international units (IU). IUs are a unit that are used to describe the bioactivity of different compounds, including 4 vitamins: A, D, E, and C. You might be wondering why these 4 vitamins, it is because these are the ones that were chosen by those who set IUs. Most likely because these vitamins are commonly included in supplements. It would be less confusing if these units were not used. However, most supplements use IUs, IUs are not as common on food items.
For vitamin E, IUs are specific for alpha-tocopherol and adjusted for the molecular weight of the different forms (alpha-tocopherol acetate etc.). The conversion factors for converting IU to mg of alpha-tocopherol are:
0.67 for RRR-alpha-tocopherol (and its esters)
0.45 for all-rac-alpha-tocopherol (and its esters)
Here are some example calculations showing how to use these conversion factors:
Example 1
For a supplement containing 100 IU of RRR-alpha tocopherol:
100 IU X 0.67 = 67 mg alpha-tocopherol
Example 2
For a supplement containing 100 IU of all-rac-alpha tocopherol:
100 IU X 0.45 = 45 mg alpha-tocopherol7,8
References
- en.Wikipedia.org/wiki/Radica.../File:VitE.gif
- Packer L, Weber SU, Rimbach G. (2001) Molecular aspects of alpha-tocotrienol antioxidant action and cell signalling. J Nutr 131(2): 369S-373S
- en.Wikipedia.org/wiki/File:Be...%C3%A4ure2.svg
- http://lpi.oregonstate.edu/mic/vitamins/vitamin-E
- Traber MG, Elsner A, Brigelius-Floh R. (1998) Synthetic as compared with natural vitamin E is preferentially excreted as alpha-CEHC in human urine: Studies using deuterated alpha-tocopheryl acetates. FEBS Lett 437(1-2): 145-148.
- Wagner KH, Kamal-Eldin A, Elmadfa I. (2004) Gamma-tocopherol--an underestimated vitamin? Ann Nutr Metab 48(3): 169-188.
- DRI (2000) Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids.
- https://ods.od.nih.gov/factsheets/Vi...hProfessional/


