3.7: Ionic Bonding and Simple Ionic Compounds (Exercises)
- Page ID
- 15221
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Additional Exercises
- What number shell is the valence electron shell of a sodium atom? What number shell is the valence shell of a sodium ion? Explain the difference.
- What number shell is the valence electron shell of a bromine atom? What number shell is the valence shell of a bromide ion? Explain the difference between these answers and the answers to Exercise 1.
- What is the electron configuration of each ion?
- K+
- Mg2+
- F−
- S2−
- What is the electron configuration of each ion?
- Li+
- Ca2+
- Cl−
- O2−
-
- If a sodium atom were to lose two electrons, what would be the electron configuration of the resulting cation?
- Considering that electron shells are typically separated by large amounts of energy, use your answer to Exercise 5a to suggest why sodium atoms do not form a 2+ cation.
-
- If a chlorine atom were to gain two electrons, what would be the electron configuration of the resulting anion?
- Considering that electron shells are typically separated by large amounts of energy, use your answer to Exercise 6a to suggest why chlorine atoms do not form a 2− anion.
- Use Lewis diagrams and arrows to show the electron transfer that occurs during the formation of an ionic compound among Mg atoms and F atoms. (Hint: how many atoms of each will you need?)
- Use Lewis diagrams and arrows to show the electron transfer that occurs during the formation of an ionic compound among K atoms and O atoms. (Hint: how many atoms of each will you need?)
- Mercury forms two possible cations—Hg2+ and Hg22+, the second of which is actually a two-atom cation with a 2+ charge.
- Using common names, give the probable names of these ions.
- What are the chemical formulas of the ionic compounds these ions make with the oxide ion, O2−?
- The uranyl ion (UO22+) is a common water-soluble form of uranium. What is the chemical formula of the ionic compound uranyl nitrate? What is the chemical formula of the ionic compound uranyl phosphate?
- The formal chemical name of the mineral strengite is iron(III) phosphate dihydrate. What is the chemical formula of strengite? What is the formula mass of strengite?
- What is the formula mass of MgSO4·7H2O?
- What is the formula mass of CaSO4·½H2O?
- What mass does 20 formula units of NaCl have?
- What mass does 75 formula units of K2SO4 have?
- If an atomic mass unit equals 1.66 × 10−24 g, what is the mass in grams of one formula unit of NaCl?
- If an atomic mass unit equals 1.66 × 10−24 g, what is the mass in grams of 5.00 × 1022 formula units of NaOH?
- If an atomic mass unit equals 1.66 × 10−24 g, what is the mass in grams of 3.96 × 1023 formula units of (NH4)2SO4?
- Both tin and lead acquire 2+ or 4+ charges when they become ions. Use the periodic table to explain why this should not surprise you.
- Which ion would you expect to be larger in size—In3+ or Tl3+? Explain.
- Which ion would you expect to be smaller in size—I− or Br−? Explain.
- Which ion with a 2+ charge has the following electron configuration? 1s22s22p6
- Which ion with a 3− charge has the following electron configuration? 1s22s22p6
Answers
- For sodium, the valence shell is the third shell; for the sodium ion, the valence shell is the second shell because it has lost all its third shell electrons.
- The valence shell for both bromine atom and bromide ion is n=4. This is because the valence shell of bromine atom can accommodate one more electron.
-
- 1s22s22p63s23p6
- 1s22s22p6
- 1s22s22p6
- 1s22s22p63s23p6
-
- 1s2
- 1s22s22p63s23p6
- 1s22s22p63s23p6
- 1s22s22p6
-
- 1s22s22p5
- It probably requires too much energy to form.
- 1. 1s22s22p63s23p64s1
2. Gaining the second electron would probably require too much energy.
7.
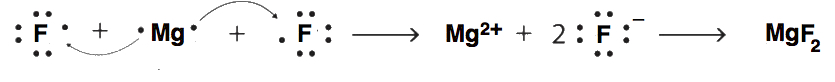
8.
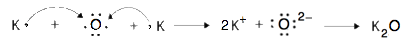
9. 1. mercuric and mercurous, respectively
2. HgO and Hg2O, respectively
10. uranyl nitrate UO2(NO3)2 and uranyl phosphate (UO2)3(PO4)2
11. FePO4·2H2O; 186.86 u
12. 246.51 u
13. 145.16 u
14. 1,169 u
15. 13,070 u
16. 9.701 x 10-23 g
17. 3.32 g
18. 86.9 g
19. Both tin and lead have two p electrons and two s electrons in their valence shells.
20. Tl3+ is larger because it is found lower on the periodic table
21. Br− because it is higher up on the periodic table
22. Mg2+
23. N3−

