10.2: Arrhenius Definition of Acids and Bases
- Page ID
- 15280
- To recognize a compound as an Arrhenius acid or an Arrhenius base.
- To describe characteristics of acids and bases.
- To write equations of neutralization reactions.
One way to define a class of compounds is by describing the various characteristics its members have in common. In the case of the compounds known as acids, the common characteristics include a sour taste, the ability to change the color of the vegetable dye litmus to red, and the ability to dissolve certain metals and simultaneously produce hydrogen gas. For the compounds called bases, the common characteristics are a slippery texture, a bitter taste, and the ability to change the color of litmus to blue. Acids and bases also react with each other to form compounds generally known as salts.
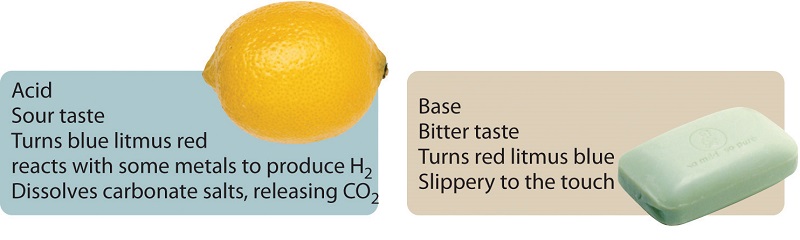
Although we include their tastes among the common characteristics of acids and bases, we never advocate tasting an unknown chemical!
Chemists prefer, however, to have definitions for acids and bases in chemical terms. The Swedish chemist Svante Arrhenius developed the first chemical definitions of acids and bases in the late 1800s. Arrhenius defined an acid as a compound that increases the concentration of hydrogen ion (H+) in aqueous solution. Many acids are simple compounds that release a hydrogen cation into solution when they dissolve. Similarly, Arrhenius defined a base as a compound that increases the concentration of hydroxide ion (OH−) in aqueous solution. Many bases are ionic compounds that have the hydroxide ion as their anion, which is released when the base dissolves in water.
| Acids | Bases | ||
|---|---|---|---|
| Formula | Name | Formula | Name |
| HCl(aq) | hydrochloric acid | NaOH(aq) | sodium hydroxide |
| HBr(aq) | hydrobromic acid | KOH(aq) | potassium hydroxide |
| HI(aq) | hydriodic acid | Mg(OH)2(aq) | magnesium hydroxide |
| H2S(aq) | hydrosulfuric acid | Ca(OH)2(aq) | calcium hydroxide |
| HC2H3O2(aq) | acetic acid | NH3(aq) | ammonia |
| HNO3(aq) | nitric acid | NaHCO3 (aq) | sodium bicarbonate |
| HNO2(aq) | nitrous acid | CaCO3 (aq) | calcium carbonate |
| H2SO4(aq) | sulfuric acid | ||
| H2SO3(aq) | sulfurous acid | ||
| HClO3(aq) | chloric acid | ||
| HClO4(aq) | perchloric acid | ||
| HClO2(aq) | chlorous acid | ||
| H3PO4(aq) | phosphoric acid | ||
| H3PO3(aq) | phosphorous acid | ||
| H2CO3(aq) | carbonic acid | ||
Many bases and their aqueous solutions are named using the normal rules of ionic compounds that were presented previously; that is, they are named as hydroxide compounds. For example, the base sodium hydroxide (NaOH) is both an ionic compound and an aqueous solution. However, aqueous solutions of acids have their own naming rules. The names of binary acids (compounds with hydrogen and one other element in their formula) are based on the root of the name of the other element preceded by the prefix hydro- and followed by the suffix -ic acid. Thus, an aqueous solution of HCl [designated “HCl(aq)”] is called hydrochloric acid, H2S(aq) is called hydrosulfuric acid, and so forth. Acids composed of more than two elements (typically hydrogen and oxygen and some other element) have names based on the name of the other element, followed by the suffix -ic acid or -ous acid, depending on the number of oxygen atoms in the acid’s formula. Other prefixes, like per- and hypo-, also appear in the names for some acids. Unfortunately, there is no strict rule for the number of oxygen atoms that are associated with the -ic acid suffix; the names of these acids are best memorized. Table \(\PageIndex{1}\) lists some acids and bases and their names. Note that acids have hydrogen written first, as if it were the cation, while most bases have the negative hydroxide ion, if it appears in the formula, written last.
The name oxygen comes from the Latin meaning “acid producer” because its discoverer, Antoine Lavoisier, thought it was the essential element in acids. Lavoisier was wrong, but it is too late to change the name now.
Name each substance.
- HF(aq)
- Sr(OH)2(aq)
Solution
- This acid has only two elements in its formula, so its name includes the hydro- prefix. The stem of the other element’s name, fluorine, is fluor, and we must also include the -ic acid ending. Its name is hydrofluoric acid.
- This base is named as an ionic compound between the strontium ion and the hydroxide ion: strontium hydroxide.
Name each substance.
- H2Se(aq)
- Ba(OH)2(aq)
- Answer
-
a. hydroselenic acid
b. barium hydroxide
Notice that one base listed in Table \(\PageIndex{1}\)—ammonia—does not have hydroxide as part of its formula. How does this compound increase the amount of hydroxide ion in aqueous solution? Instead of dissociating into hydroxide ions, ammonia molecules react with water molecules by taking a hydrogen ion from the water molecule to produce an ammonium ion and a hydroxide ion:
\[NH_{3(aq)} + H_2O_{(ℓ)} \rightarrow NH^+_{4(aq)} + OH^−_{(aq)} \label{Eq1} \]
Because this reaction of ammonia with water causes an increase in the concentration of hydroxide ions in solution, ammonia satisfies the Arrhenius definition of a base. Many other nitrogen-containing compounds are bases because they too react with water to produce hydroxide ions in aqueous solution.
Neutralization
As we noted previously, acids and bases react chemically with each other to form salts. A salt is a general chemical term for any ionic compound formed from an acid and a base. In reactions where the acid is a hydrogen ion containing compound and the base is a hydroxide ion containing compound, water is also a product. The general reaction is as follows:
acid + base → water + salt
The reaction of acid and base to make water and a salt is called neutralization. Like any chemical equation, a neutralization chemical equation must be properly balanced. For example, the neutralization reaction between sodium hydroxide and hydrochloric acid is as follows:
\[NaOH{(aq)} + HCl_{(aq)} \rightarrow NaCl_{(aq)} + H_2O_{(ℓ)} \label{Eq2} \]
with coefficients all understood to be one. The neutralization reaction between sodium hydroxide and sulfuric acid is as follows:
\[2NaOH_{(aq)} + H_2SO_{4(aq)} \rightarrow Na_2SO_{4(aq)} + 2H_2O_{(ℓ)} \label{Eq3} \]
Once a neutralization reaction is properly balanced, we can use it to perform stoichiometry calculations, such as the ones we practiced earlier.
There are a number of examples of acid-base chemistry in everyday life. One example is the use of baking soda, or sodium bicarbonate in baking. NaHCO3 is a base. When it reacts with an acid such as lemon juice, buttermilk, or sour cream in a batter, bubbles of carbon dioxide gas are formed from decomposition of the resulting carbonic acid, and the batter “rises.” Baking powder is a combination of sodium bicarbonate, and one or more acid salts that react when the two chemicals come in contact with water in the batter.
\[ HCO_3^- (aq) + H^+ (aq) \rightarrow H_2 CO_3 (aq) \label{4.3.19} \]
\[ H_2 CO_3 (aq) \rightarrow CO_2 (g) + H_2 O(l) \nonumber \]
Nitric acid [HNO3(aq)] can be neutralized by calcium hydroxide [Ca(OH)2(aq)].
- Write a balanced chemical equation for the reaction between these two compounds and identify the salt it produces.
- For one reaction, 16.8 g of HNO3 is present initially. How many grams of Ca(OH)2 are needed to neutralize that much HNO3?
- In a second reaction, 805 mL of 0.672 M Ca(OH)2 is present initially. What volume of 0.432 M HNO3 solution is necessary to neutralize the Ca(OH)2 solution?
Solution
- Because there are two OH− ions in the formula for Ca(OH)2, we need two moles of HNO3 to provide H+ ions. The balanced chemical equation is as follows: Ca(OH)2(aq) + 2HNO3(aq) → Ca(NO3)2(aq) + 2H2O(ℓ)
The salt formed is calcium nitrate.
- This calculation is much like the calculations we did in Chapter 6. First we convert the mass of HNO3 to moles using its molar mass of 1.01 + 14.01 + 3(16.00) = 63.02 g/mol; then we use the balanced chemical equation to determine the related number of moles of Ca(OH)2 needed to neutralize it; and then we convert that number of moles of Ca(OH)2 to the mass of Ca(OH)2 using its molar mass of 40.08 + 2(1.01) + 2(16.00) = 74.10 g/mol.
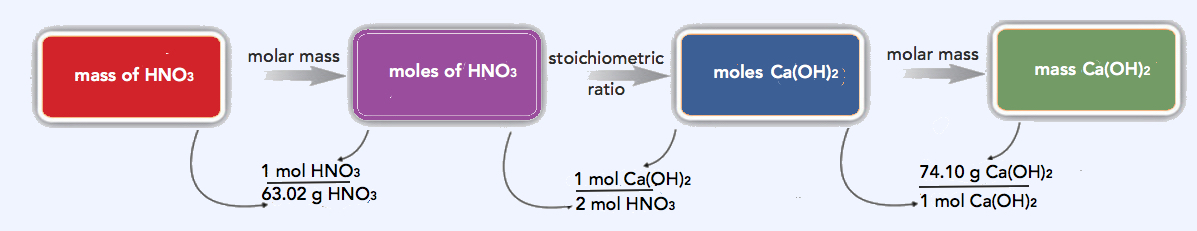
\(\mathrm{16.8\: g\: HNO_3 \times \dfrac{1\: mol\: HNO_3}{63.02\: g\ HNO_3} \times \dfrac{1\: mol\: Ca(OH)_2}{2\: mol\: HNO_3} \times \dfrac{74.10\: g\: Ca(OH)_2}{1\: mol\: Ca(OH)_2}=9.88\: g\: Ca(OH)_2\: needed}\)
- Having concentration information allows us to employ the skills we developed in Chapter 9. We have two alternative solutions: the multi-step process and the combined one-line process (found at the bottom).
First, we use the concentration and volume data to determine the number of moles of Ca(OH)2 present. Recognizing that 805 mL = 0.805 L,
\(\mathrm{0.672\: M\: Ca(OH)_2=\dfrac{mol\: Ca(OH)_2}{0.805\: L\: soln}}\)
0.672 M CaOH)2 × (0.805 L soln) = mol Ca(OH)2 = 0.541 mol Ca(OH)2
We combine this information with the proper ratio from the balanced chemical equation to determine the number of moles of HNO3 needed:
\(\mathrm{0.541\: mol\: Ca(OH)_2 \times \dfrac{2\: mol\: HNO_3}{1\: mol\: Ca(OH)_2}=1.08\: mol\: HNO_3}\)
Now, using the definition of molarity one more time, we determine the volume of acid solution needed:
\(\mathrm{0.432\: M\: HNO_3=\dfrac{1.08\: mol\: HNO_3}{volume\: of\: HNO_3}}\)
\(\mathrm{volume\: of\: HNO_3=\dfrac{1.08\: mol\: HNO_3}{0.432\: M\: HNO_3}=2.50\: L=2.50\times10^3\: mL\: HNO_3}\)
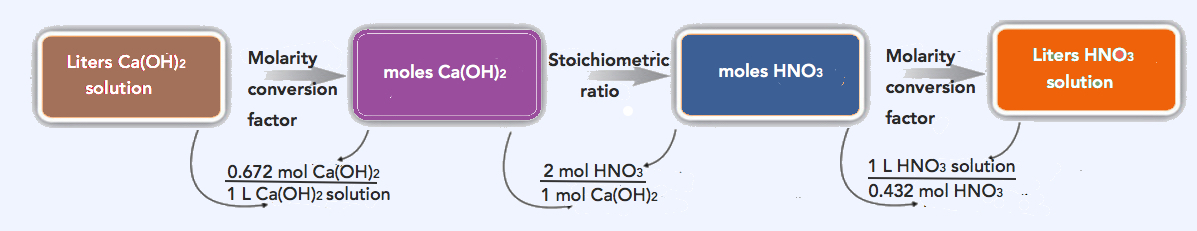
\(\mathrm{0.805\: L\: Ca(OH)_2\: soln \times \dfrac{0.672\: mol\: Ca(OH)_2 }{1\: L\ Ca(OH)_2\: soln } \times \dfrac{2\: mol\: HNO_3}{1\: mol\: Ca(OH)_2} \times \dfrac{1\: L\: HNO_3\: soln}{0.432\: mol\: HNO_3}=2.50\: L\: HNO_3\: soln\: needed}\)
Hydrocyanic acid [HCN(aq)] can be neutralized by potassium hydroxide [KOH(aq)].
- Write a balanced chemical equation for the reaction between these two compounds and identify the salt it produces.
- For one reaction, 37.5 g of HCN is present initially. How many grams of KOH are needed to neutralize that much HCN?
- In a second reaction, 43.0 mL of 0.0663 M KOH is present initially. What volume of 0.107 M HCN solution is necessary to neutralize the KOH solution?
- Answer
-
a. KOH(aq) + HCN(aq) → KCN(aq) + H2O(ℓ) KCN is the salt.
b. 77.8 g
c. 0.0266 L or 26.6 mL
Hydrocyanic acid (HCN) is one exception to the acid-naming rules that specify using the prefix hydro- for binary acids (acids composed of hydrogen and only one other element).
Stomach Antacids
Our stomachs contain a solution of roughly 0.03 M HCl, which helps us digest the food we eat. The burning sensation associated with heartburn is a result of the acid of the stomach leaking through the muscular valve at the top of the stomach into the lower reaches of the esophagus. The lining of the esophagus is not protected from the corrosive effects of stomach acid the way the lining of the stomach is, and the results can be very painful. When we have heartburn, it feels better if we reduce the excess acid in the esophagus by taking an antacid. As you may have guessed, antacids are bases. One of the most common antacids is calcium carbonate, CaCO3. The reaction,
\[CaCO_3(s)+2HCl(aq)⇌CaCl_2(aq)+H_2O(l)+CO_2(g) \nonumber \]
not only neutralizes stomach acid, it also produces CO2(g), which may result in a satisfying belch.
Milk of Magnesia is a suspension of the sparingly soluble base magnesium hydroxide, Mg(OH)2. It works according to the reaction:
\[Mg(OH)_2(s)⇌Mg^{2+}(aq)+2OH^-(aq) \nonumber \]
The hydroxide ions generated in this equilibrium then go on to react with the hydronium ions from the stomach acid, so that :
\[H_3O^+ + OH^- ⇌ 2H_2O(l) \nonumber \]
This reaction does not produce carbon dioxide, but magnesium-containing antacids can have a laxative effect. Several antacids have aluminum hydroxide, Al(OH)3, as an active ingredient. The aluminum hydroxide tends to cause constipation, and some antacids use aluminum hydroxide in concert with magnesium hydroxide to balance the side effects of the two substances.
Assume that the stomach of someone suffering from acid indigestion contains 75 mL of 0.20 M HCl. How many Tums tablets are required to neutralize 90% of the stomach acid, if each tablet contains 500 mg of CaCO3? (Neutralizing all of the stomach acid is not desirable because that would completely shut down digestion.)
Solution
- Write the balanced chemical equation for the reaction and then decide whether the reaction will go to completion.
- Calculate the number of moles of acid present. Multiply the number of moles by the percentage to obtain the quantity of acid that must be neutralized. Using mole ratios, calculate the number of moles of base required to neutralize the acid.
- Calculate the number of moles of base contained in one tablet by dividing the mass of base by the corresponding molar mass. Calculate the number of tablets required by dividing the moles of base by the moles contained in one tablet.
A. We first write the balanced chemical equation for the reaction:
\(2HCl(aq) + CaCO_3(s) \rightarrow CaCl_2(aq) + H_2CO_3(aq)\)
Each carbonate ion can react with 2 mol of H+ to produce H2CO3, which rapidly decomposes to H2O and CO2. Because HCl is a strong acid and CO32− is a weak base, the reaction will go to completion.
B. Next we need to determine the number of moles of HCl present:
\( 75\: \cancel{mL} \left( \dfrac{1\: \cancel{L}} {1000\: \cancel{mL}} \right) \left( \dfrac{0 .20\: mol\: HCl} {\cancel{L}} \right) = 0. 015\: mol\: HCl \)
Because we want to neutralize only 90% of the acid present, we multiply the number of moles of HCl by 0.90:
\((0.015\: mol\: HCl)(0.90) = 0.014\: mol\: HCl\)
We know from the stoichiometry of the reaction that each mole of CaCO3 reacts with 2 mol of HCl, so we need
\( moles\: CaCO_3 = 0 .014\: \cancel{mol\: HCl} \left( \dfrac{1\: mol\: CaCO_3}{2\: \cancel{mol\: HCl}} \right) = 0 .0070\: mol\: CaCO_3 \)
C. Each Tums tablet contains
\( \left( \dfrac{500\: \cancel{mg\: CaCO_3}} {1\: Tums\: tablet} \right) \left( \dfrac{1\: \cancel{g}} {1000\: \cancel{mg\: CaCO_3}} \right) \left( \dfrac{1\: mol\: CaCO_3} {100 .1\: \cancel{g}} \right) = 0 .00500\: mol\: CaCO_ 3 \)
Thus we need \(\dfrac{0.0070\: \cancel{mol\: CaCO_3}}{0.00500\: \cancel{mol\: CaCO_3}}= 1.4\) Tums tablets.
Exercise \(\PageIndex{3}\)
Assume that as a result of overeating, a person’s stomach contains 300 mL of 0.25 M HCl. How many Rolaids tablets must be consumed to neutralize 95% of the acid, if each tablet contains 400 mg of NaAl(OH)2CO3? The neutralization reaction can be written as follows:
\( NaAl(OH)_2CO_3(s) + 4HCl(aq) \rightarrow AlCl_3(aq) + NaCl(aq) + CO_2(g) + 3H_2O(l) \)
- Answer
-
6.4 tablets
Key Takeaway
- An Arrhenius acid increases the H+ ion concentration in water, while an Arrhenius base increases the OH− ion concentration in water.

