10.3: Brønsted-Lowry Definition of Acids and Bases
- Page ID
- 15281
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Recognize a compound as a Brønsted-Lowry acid or a Brønsted-Lowry base.
- Illustrate the proton transfer process that defines a Brønsted-Lowry acid-base reaction.
Ammonia (NH3) increases the hydroxide ion concentration in aqueous solution by reacting with water rather than releasing hydroxide ions directly. In fact, the Arrhenius definitions of an acid and a base focus on hydrogen ions and hydroxide ions. Are there more fundamental definitions for acids and bases?
In 1923, the Danish scientist Johannes Brønsted and the English scientist Thomas Lowry independently proposed new definitions for acids and bases. Rather than considering both hydrogen and hydroxide ions, they focused on the hydrogen ion only. A Brønsted-Lowry acid is a compound that supplies a hydrogen ion in a reaction. A Brønsted-Lowry base, conversely, is a compound that accepts a hydrogen ion in a reaction. Thus, the Brønsted-Lowry definitions of an acid and a base focus on the movement of hydrogen ions in a reaction, rather than on the production of hydrogen ions and hydroxide ions in an aqueous solution.
Let us use the reaction of ammonia in water to demonstrate the Brønsted-Lowry definitions of an acid and a base. Ammonia and water molecules are reactants, while the ammonium ion and the hydroxide ion are products:
\[NH_{3}(aq) + H_2O(ℓ) \rightarrow NH^+_{4}(aq) + OH^−(aq) \label{Eq1} \]
What has happened in this reaction is that the original water molecule has donated a hydrogen ion to the original ammonia molecule, which in turn has accepted the hydrogen ion. We can illustrate this as follows:
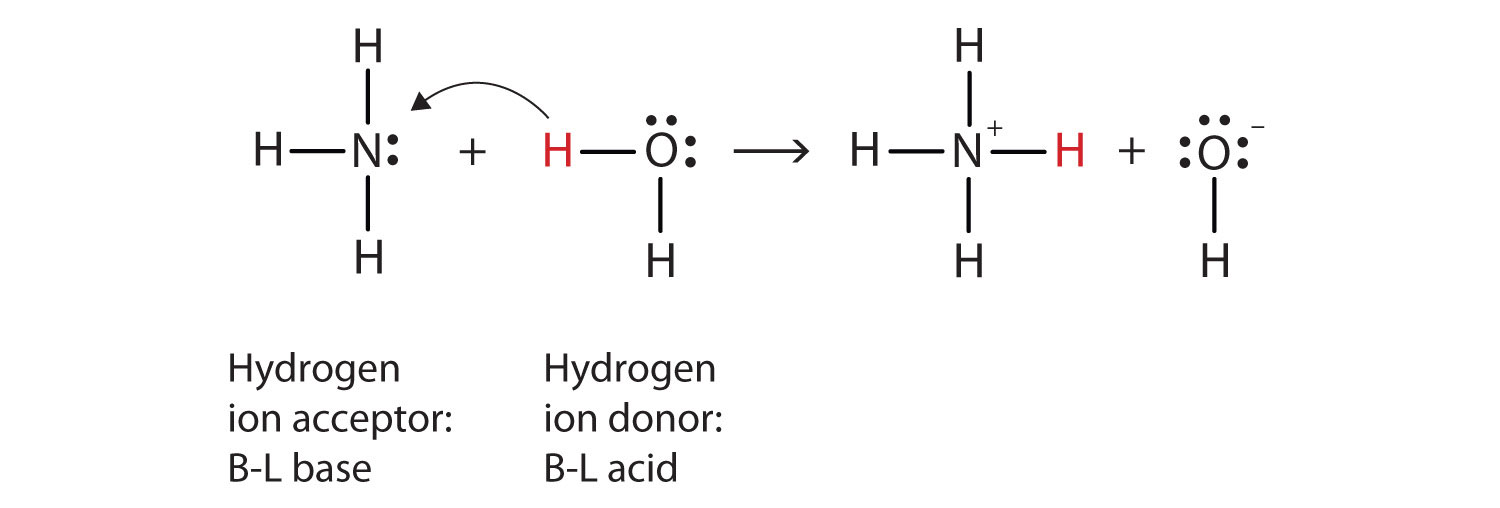
Because the water molecule donates a hydrogen ion to the ammonia, it is the Brønsted-Lowry acid, while the ammonia molecule—which accepts the hydrogen ion—is the Brønsted-Lowry base. Thus, ammonia acts as a base in both the Arrhenius sense and the Brønsted-Lowry sense.
Is an Arrhenius acid like hydrochloric acid still an acid in the Brønsted-Lowry sense? Yes, but it requires us to understand what really happens when HCl is dissolved in water. Recall that the hydrogen atom is a single proton surrounded by a single electron. To make the hydrogen ion, we remove the electron, leaving a bare proton. Do we really have bare protons floating around in aqueous solution? No, we do not. What really happens is that the H+ ion attaches itself to H2O to make H3O+, which is called the hydronium ion. For most purposes, H+ and H3O+ represent the same species, but writing H3O+ instead of H+ shows that we understand that there are no bare protons floating around in solution. Rather, these protons are actually attached to solvent molecules.
A proton in aqueous solution may be surrounded by more than one water molecule, leading to formulas like H5O2+ or H9O4+ rather than H3O+. It is simpler, however, to use H3O+.
With this in mind, how do we define HCl as an acid in the Brønsted-Lowry sense? Consider what happens when HCl is dissolved in H2O:
\[HCl(g) + H_2O(ℓ) \rightarrow H_3O^+(aq) + Cl^−(aq) \label{Eq2} \]
We can depict this process using Lewis electron dot diagrams:
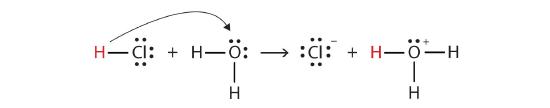
Now we see that a hydrogen ion is transferred from the HCl molecule to the H2O molecule to make chloride ions and hydronium ions. As the hydrogen ion donor, HCl acts as a Brønsted-Lowry acid; as a hydrogen ion acceptor, H2O is a Brønsted-Lowry base. So HCl is an acid not just in the Arrhenius sense but also in the Brønsted-Lowry sense. Moreover, by the Brønsted-Lowry definitions, H2O is a base in the formation of aqueous HCl. So the Brønsted-Lowry definitions of an acid and a base classify the dissolving of HCl in water as a reaction between an acid and a base—although the Arrhenius definition would not have labeled H2O a base in this circumstance.
All Arrhenius acids and bases are Brønsted-Lowry acids and bases as well. However, not all Brønsted-Lowry acids and bases are Arrhenius acids and bases.
Aniline (C6H5NH2) is slightly soluble in water. It has a nitrogen atom that can accept a hydrogen ion from a water molecule just like the nitrogen atom in ammonia does. Write the chemical equation for this reaction and identify the Brønsted-Lowry acid and base.
Solution
C6H5NH2 and H2O are the reactants. When C6H5NH2 accepts a proton from H2O, it gains an extra H and a positive charge and leaves an OH− ion behind. The reaction is as follows:
C6H5NH2(aq) + H2O(ℓ) → C6H5NH3+(aq) + OH−(aq)
Because C6H5NH2 accepts a proton, it is the Brønsted-Lowry base. The H2O molecule, because it donates a proton, is the Brønsted-Lowry acid.
Caffeine (C8H10N4O2) is a stimulant found in coffees and teas. When dissolved in water, it can accept a proton from a water molecule. Write the chemical equation for this process and identify the Brønsted-Lowry acid and base.
- Answer
-
C8H10N4O2(aq) + H2O(ℓ) → C8H11N4O2+(aq) + OH−(aq)
B-L base B-L acid
The Brønsted-Lowry definitions of an acid and a base can be applied to chemical reactions that occur in solvents other than water. The following example illustrates.
Sodium amide (NaNH2) dissolves in methanol (CH3OH) and separates into sodium ions and amide ions (NH2−). The amide ions react with methanol to make ammonia and the methoxide ion (CH3O−). Write a balanced chemical equation for this process and identify the Brønsted-Lowry acid and base.
Solution
The equation for the reaction is between NH2− and CH3OH to make NH3 and CH3O− is as follows:
NH2−(solv) + CH3OH(ℓ) → NH3(solv) + CH3O−(solv)
The label (solv) indicates that the species are dissolved in some solvent, in contrast to (aq), which specifies an aqueous (H2O) solution. In this reaction, we see that the NH2− ion accepts a proton from a CH3OH molecule to make an NH3 molecule. Thus, as the proton acceptor, NH2− is the Brønsted-Lowry base. As the proton donor, CH3OH is the Brønsted-Lowry acid.
Pyridinium chloride (C5H5NHCl) dissolves in ethanol (C2H5OH) and separates into pyridinium ions (C5H5NH+) and chloride ions. The pyridinium ion can transfer a hydrogen ion to a solvent molecule. Write a balanced chemical equation for this process and identify the Brønsted-Lowry acid and base.
- Answer
-
C5H5NH+(solv) + C2H5OH(ℓ) → C5H5N(solv) + C2H5OH2+(solv)
B-L acid B-L base
Application in Everyday Life
Many people like to put lemon juice or vinegar, both of which are acids, on cooked fish (Figure \(\PageIndex{1}\)). It turns out that fish have volatile amines (bases) in their systems, which are neutralized by the acids to yield involatile ammonium salts. This reduces the odor of the fish, and also adds a “sour” taste that we seem to enjoy.
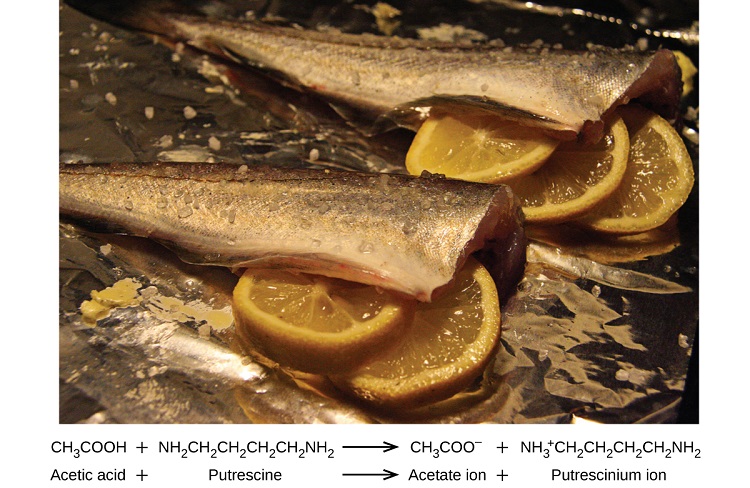 Figure \(\PageIndex{1}\): A neutralization reaction takes place between citric acid or acetic acid (proton donors) in lemons or vinegar and putrescine (proton acceptor) in the flesh of fish. (CC BY 4.0; OpenStax)
Figure \(\PageIndex{1}\): A neutralization reaction takes place between citric acid or acetic acid (proton donors) in lemons or vinegar and putrescine (proton acceptor) in the flesh of fish. (CC BY 4.0; OpenStax)
Pickling is a method used to preserve vegetables using a naturally produced acidic environment. The vegetable, such as a cucumber, is placed in a sealed jar submerged in a brine solution. The brine solution favors the growth of beneficial bacteria and suppresses the growth of harmful bacteria. The beneficial bacteria feed on starches in the cucumber and produce lactic acid as a waste product in a process called fermentation. The lactic acid eventually increases the acidity of the brine to a level that kills any harmful bacteria, which require a basic environment. Without the harmful bacteria consuming the cucumbers they are able to last much longer than if they were unprotected. A byproduct of the pickling process changes the flavor of the vegetables with the acid making them taste sour.
To Your Health: Brønsted-Lowry Acid-Base Reactions in Pharmaceuticals
There are many interesting applications of Brønsted-Lowry acid-base reactions in the pharmaceutical industry. For example, drugs often need to be water soluble for maximum effectiveness. However, many complex organic compounds are not soluble or are only slightly soluble in water. Fortunately, those drugs that contain proton-accepting nitrogen atoms (and there are a lot of them) can be reacted with dilute hydrochloric acid [HCl(aq)]. The nitrogen atoms—acting as Brønsted-Lowry bases—accept the hydrogen ions from the acid to make an ion, which is usually much more soluble in water. The modified drug molecules can then be isolated as chloride salts:
\[\mathrm{RN(sl\: aq) + H^+(aq) \rightarrow RNH^+(aq) \xrightarrow{Cl^-(aq)} RNHCl(s)} \label{Eq3} \]
where RN represents some organic compound containing nitrogen. The label (sl aq) means “slightly aqueous,” indicating that the compound RN is only slightly soluble. Drugs that are modified in this way are called hydrochloride salts. Examples include the powerful painkiller codeine, which is commonly administered as codeine hydrochloride. Acids other than hydrochloric acid are also used. Hydrobromic acid, for example, gives hydrobromide salts. Dextromethorphan, an ingredient in many cough medicines, is dispensed as dextromethorphan hydrobromide. The accompanying figure shows another medication (lidocaine) as a hydrochloride salt.
 Figure used with permission from Wikipedia.
Figure used with permission from Wikipedia.
Conjugate Acid-Base Pairs
According to the Bronsted-Lowry theory of acids and bases, an acid is a proton donor and a base is a proton acceptor. Once an acid has given up a proton, the part that remains is called the acid's conjugate base. This species is a base because it can accept a proton (to re-form the acid). The conjugate base of HF (first example below) is fluoride ion, F-.
\(\mathrm{{\color{Red} Acid} = H^+ + {\color{Blue} Conjugate\: base\: of\: Acid}^-}\)
\({\color{Red} \mathrm{HF}} \rightleftharpoons \mathrm{H^+} + {\color{Blue} \mathrm{F^-}}\)
\({\color{Red} \mathrm{H_2O}} \rightleftharpoons \mathrm{H^+} + {\color{Blue} \mathrm{OH^-}}\)
\({\color{Red} \mathrm{NH_4^+}} \rightleftharpoons \mathrm{H^+} + {\color{Blue} \mathrm{NH_3}}\)
Similarly, the part of the base that remains after a base accepts a proton is called the base's conjugate acid. This species is an acid because it can give up a proton (and thus re-form the base). The conjugate acid of fluoride ion, F- (first example below) is HF.
\(\mathrm{H^+ + {\color{Blue} Base} = {\color{Red} Conjugate\: acid\: of\: Base}^+}\)
\(\mathrm{H^+ + {\color{Blue} F^- } \rightleftharpoons {\color{Red} HF}}\)
\(\mathrm{H^+ + {\color{Blue} OH^- } \rightleftharpoons {\color{Red} H_2O}}\)
\(\mathrm{H^+ + {\color{Blue} H_2O } \rightleftharpoons {\color{Red} H_3O^+}}\)
\(\mathrm{H^+ + {\color{Blue} NH_3 } \rightleftharpoons {\color{Red} NH_4^+}}\)
To summarize, the conjugate base of HF is fluoride ion, F-, and the conjugate acid of fluoride ion, F-, is HF. The HF/F- pair is referred to as a conjugate acid-base pair. The difference in the formulas of a conjugate acid-base pair (example: HF and F-) is H+. The table below lists conjugate acid-base pairs for your reference so that you can figure out the strategy of identifying them. For any given acid or base, you should be able to give its conjugate base or conjugate acid. The formula of an acid's conjugate base is generated by removing a proton (H+) from the acid formula. The formula of the base's conjugate acid is formed by adding a proton (H+) to the formula of the base.
| Conjugate Acid | Conjugate Base |
|---|---|
| \(\ce{H3O+}\) | \(\ce{H2O}\) |
| \(\ce{H2O}\) | \(\ce{OH-}\) |
| \(\ce{H2SO4}\) | \(\ce{HSO4-}\) |
| \(\ce{HSO4-}\) | \(\ce{SO4^2-}\) |
| \(\ce{NH4+}\) | \(\ce{NH3}\) |
| \(\ce{NH3}\) | \(\ce{NH2-}\) |
| \(\ce{CH3COOH}\) | \(\ce{CH3COO-}\) |
| \(\ce{CH3NH3+}\) | \(\ce{CH3NH2}\) |
Write the formula of the conjugate base of (a) HCl and (b) HCO3– .
Write the formula of the conjugate acid of (c) CH3NH2and (d) OH–.
Solution:
A conjugate base is formed by removing a proton (H+). A conjugate acid is formed by accepting a proton (H+).
- After HCl donates a proton, a Cl– ion is produced, and so Cl– is the conjugate base.
- After hydrogen carbonate ion, HCO3–, donates a proton, its conjugate base, CO32– is produced.
- After accepting a proton (H+), CH3NH2 is converted to CH3NH3+, its conjugate acid.
- After accepting a proton (H+), OH– is converted to H2O, its conjugate acid.
Exercise \(\PageIndex{3}\): Conjugate Pairs
Write the formula of the conjugate base of (a) HNO2 and (b) H2CO3.
Write the formula of the conjugate acid of (c) C6H5NH2and (d) HCO3–.
- Answer
-
a. NO2– is the conjugate base of HNO2.
b. HCO3– is the conjugate base of H2CO3
c. C6H5NH3+ is the conjugate acid of C6H5NH2.
d. H2CO3is the conjugate acid ofHCO3–
In the reaction of ammonia with water to give ammonium ions and hydroxide ions, ammonia acts as a base by accepting a proton from a water molecule, which in this case means that water is acting as an acid. In the reverse reaction, an ammonium ion acts as an acid by donating a proton to a hydroxide ion, and the hydroxide ion acts as a base. The conjugate acid–base pairs for this reaction are \(NH_4^+/NH_3\) and \(H_2O/OH^−\). This means that the conjugate acid of the base NH3 is NH4+ while the conjugate base of the acid NH4+ is NH3. Similarly, the conjugate base of the acid H2O is OH-, and the conjugate acid of the base OH- is H2O.
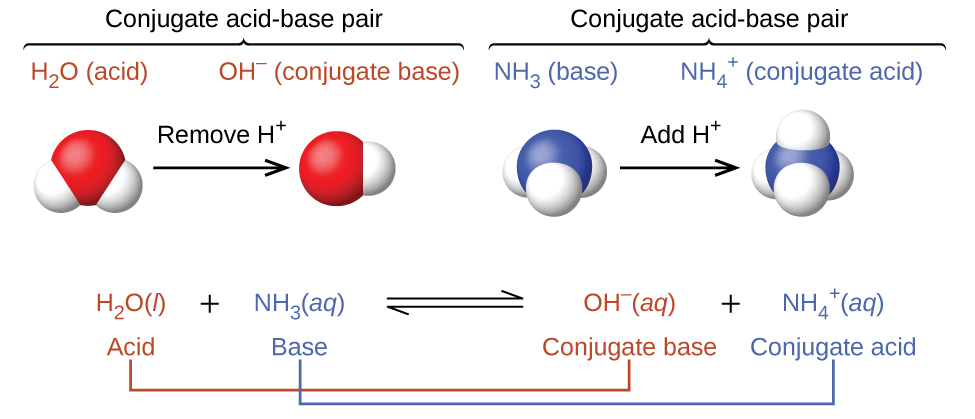 This figure has three parts in two rows. In the first row, two diagrams of acid-base pairs are shown. On the left, a space filling model of H subscript 2 O is shown with a red O atom at the center and two smaller white H atoms attached in a bent shape. Above this model is the label “H subscript 2 O (acid)” in purple. An arrow points right, which is labeled “Remove H superscript plus.” To the right is another space filling model with a single red O atom to which a single smaller white H atom is attached. The label in purple above this model reads, “O H superscript negative (conjugate base).” Above both of these red and white models is an upward pointing bracket that is labeled “Conjugate acid-base pair.” To the right is a space filling model with a central blue N atom to which three smaller white H atoms are attached in a triangular pyramid arrangement. A label in green above reads “N H subscript 3 (base).” An arrow labeled “Add H superscript plus” points right. To the right of the arrow is another space filling model with a blue central N atom and four smaller white H atoms in a tetrahedral arrangement. The green label above reads “N H subscript 3 superscript plus (conjugate acid).” Above both of these blue and white models is an upward pointing bracket that is labeled “Conjugate acid-base pair.” The second row of the figure shows the chemical reaction, H subscript 2 O ( l ) is shown in purple, and is labeled below in purple as “acid,” plus N H subscript 3 (a q) in green, labeled below in green as “base,” followed by a double sided arrow arrow and O H superscript negative (a q) in purple, labeled in purple as “conjugate base,” plus N H subscript 4 superscript plus (a q)” in green, which is labeled in green as “conjugate acid.” The acid on the left side of the equation is connected to the conjugate base on the right with a purple line. Similarly, the base on the left is connected to the conjugate acid on the right side.
This figure has three parts in two rows. In the first row, two diagrams of acid-base pairs are shown. On the left, a space filling model of H subscript 2 O is shown with a red O atom at the center and two smaller white H atoms attached in a bent shape. Above this model is the label “H subscript 2 O (acid)” in purple. An arrow points right, which is labeled “Remove H superscript plus.” To the right is another space filling model with a single red O atom to which a single smaller white H atom is attached. The label in purple above this model reads, “O H superscript negative (conjugate base).” Above both of these red and white models is an upward pointing bracket that is labeled “Conjugate acid-base pair.” To the right is a space filling model with a central blue N atom to which three smaller white H atoms are attached in a triangular pyramid arrangement. A label in green above reads “N H subscript 3 (base).” An arrow labeled “Add H superscript plus” points right. To the right of the arrow is another space filling model with a blue central N atom and four smaller white H atoms in a tetrahedral arrangement. The green label above reads “N H subscript 3 superscript plus (conjugate acid).” Above both of these blue and white models is an upward pointing bracket that is labeled “Conjugate acid-base pair.” The second row of the figure shows the chemical reaction, H subscript 2 O ( l ) is shown in purple, and is labeled below in purple as “acid,” plus N H subscript 3 (a q) in green, labeled below in green as “base,” followed by a double sided arrow arrow and O H superscript negative (a q) in purple, labeled in purple as “conjugate base,” plus N H subscript 4 superscript plus (a q)” in green, which is labeled in green as “conjugate acid.” The acid on the left side of the equation is connected to the conjugate base on the right with a purple line. Similarly, the base on the left is connected to the conjugate acid on the right side.
In the forward reaction, the parent acid is H2O and and the parent base is NH3(shown in the illustration below). The acid H2O loses a proton (H+) to form its conjugate base OH-. The base NH3 gains a proton, to produce its conjugate acid NH4+. In the reverse reaction, the acid NH4+ loses a proton (H+) to form its conjugate base NH3. The base OH- gains a proton, to produce its conjugate acid H2O.
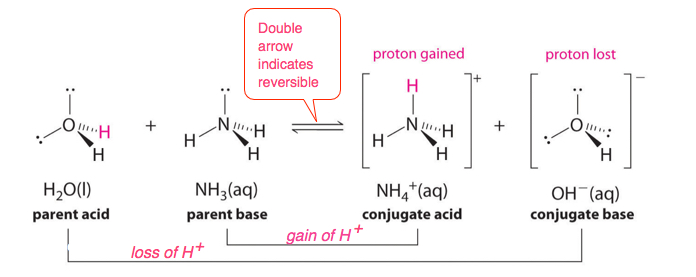
When hydrogen fluoride (HF) dissolves in water and ionizes, protons are transferred from hydrogen fluoride (parent acid) molecules to water (parent base) molecules, yielding hydronium ions (conjugate acid of water) and fluoride ions (conjugate base of HF):
\({\color{Red} \mathrm{HF}} + {\color{Blue} H_2O } \rightleftharpoons {\color{Blue} H_3O^+} + {\color{Red} \mathrm{F^-}}\)

Identify the conjugate acid-base pairs in this equilibrium.
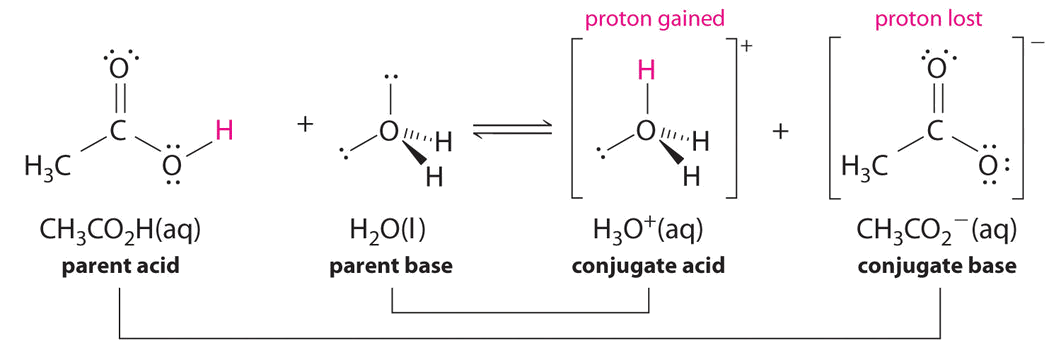
\[\ce{CH3CO2H + H2O <=> H3O^{+} + CH3CO2^{-}} \nonumber \]
Solution
Similarly, in the reaction of acetic acid with water, acetic acid donates a proton to water, which acts as the base. In the reverse reaction, \(H_3O^+\) is the acid that donates a proton to the acetate ion, which acts as the base.
Once again, we have two conjugate acid–base pairs:
- the parent acid and its conjugate base (\(CH_3CO_2H/CH_3CO_2^−\)) and
- the parent base and its conjugate acid (\(H_3O^+/H_2O\)).
Identify the conjugate acid-base pairs in this equilibrium.
\[(CH_{3})_{3}N + H_{2}O\rightleftharpoons (CH_{3})_{3}NH^{+} + OH^{-} \nonumber \]
Solution
One pair is H2O and OH−, where H2O has one more H+ and is the conjugate acid, while OH− has one less H+ and is the conjugate base.
The other pair consists of (CH3)3N and (CH3)3NH+, where (CH3)3NH+ is the conjugate acid (it has an additional proton) and (CH3)3N is the conjugate base.
Identify the conjugate acid-base pairs in this equilibrium.
\[\ce{NH2^{-} + H2O\rightleftharpoons NH3 + OH^{-}} \nonumber \]
- Answer:
- H2O (acid) and OH− (base); NH2− (base) and NH3 (acid)
The use of conjugate acid-base pairs allows us to make a very simple statement about relative strengths of acids and bases. The stronger an acid, the weaker its conjugate base, and, conversely, the stronger a base, the weaker its conjugate acid.
Key Takeaways
- A Brønsted-Lowry acid is a proton donor, and a Brønsted-Lowry base is a proton acceptor.
- Brønsted-Lowry acid-base reactions are essentially proton transfer reactions.

