18.2: Body Fluids and Fluid Compartment
- Page ID
- 57588
Learning Objectives
- Explain the importance of water in the body
- Contrast the composition of the intracellular fluid with that of the extracellular fluid
- Explain the importance of protein channels in the movement of solutes
- Identify the causes and symptoms of edema
The chemical reactions of life take place in aqueous solutions. The dissolved substances in a solution are called solutes. In the human body, solutes vary in different parts of the body, but may include proteins—including those that transport lipids, carbohydrates, and, very importantly, electrolytes. Often in medicine, a mineral dissociated from a salt that carries an electrical charge (an ion) is called and electrolyte. For instance, sodium ions (Na+) and chloride ions (Cl-) are often referred to as electrolytes.
In the body, water moves through semi-permeable membranes of cells and from one compartment of the body to another by a process called osmosis. Osmosis is basically the diffusion of water from regions of higher concentration to regions of lower concentration, along an osmotic gradient across a semi-permeable membrane. As a result, water will move into and out of cells and tissues, depending on the relative concentrations of the water and solutes found there. An appropriate balance of solutes inside and outside of cells must be maintained to ensure normal function.
Body Water Content
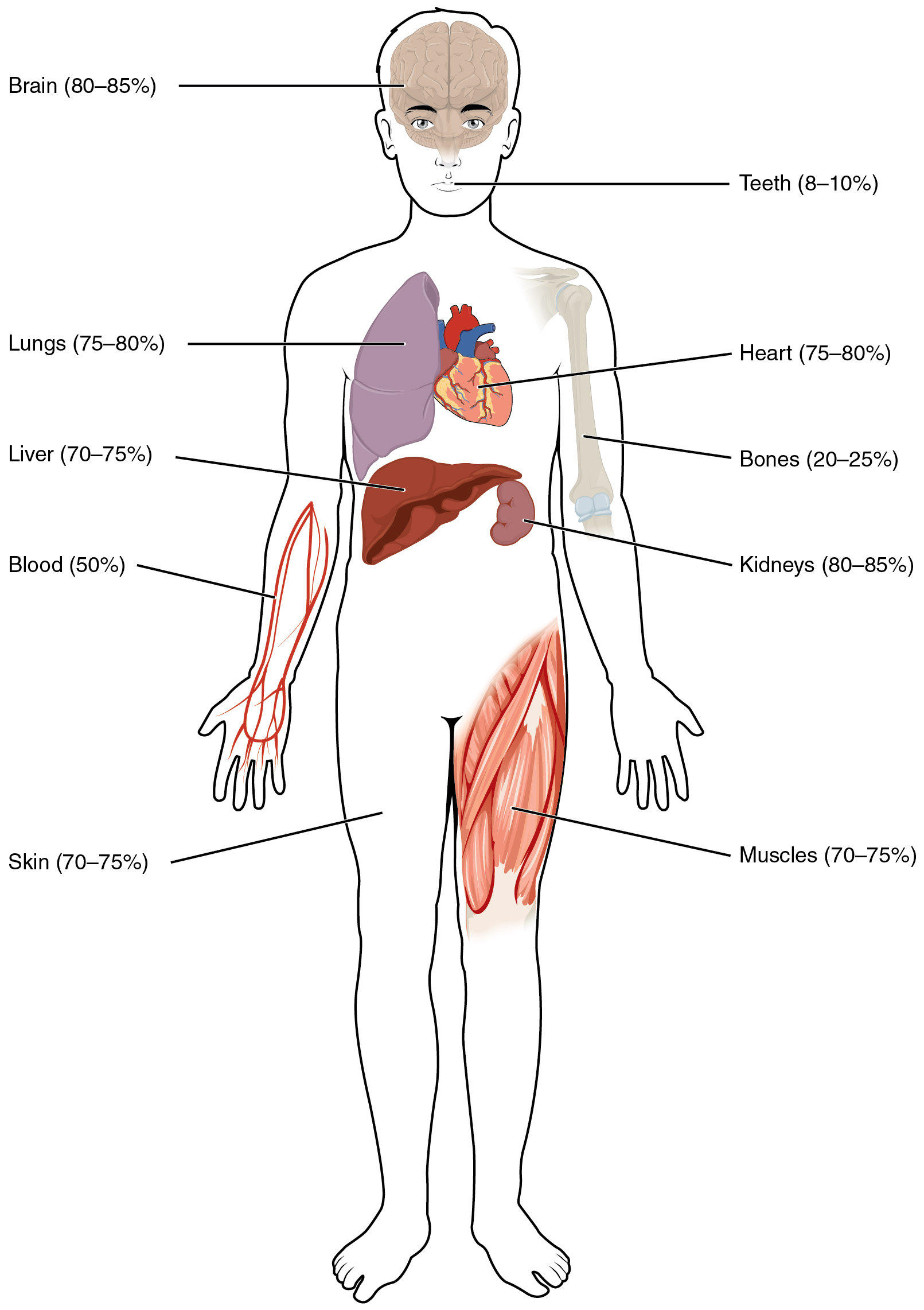
Human beings are mostly water, ranging from about 75 percent of body mass in infants to about 50–60 percent in adult men and women, to as low as 45 percent in old age. The percent of body water changes with development, because the proportions of the body given over to each organ and to muscles, fat, bone, and other tissues change from infancy to adulthood (Figure \(\PageIndex{1}\)). Your brain and kidneys have the highest proportions of water, which composes 80–85 percent of their masses. In contrast, teeth have the lowest proportion of water, at 8–10 percent.
Fluid Compartments
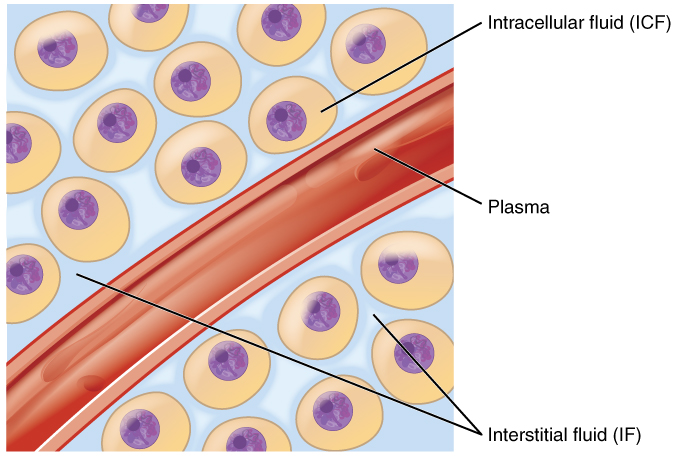
Body fluids can be discussed in terms of their specific fluid compartment, a location that is largely separate from another compartment by some form of a physical barrier. The intracellular fluid (ICF) compartment is the system that includes all fluid enclosed in cells by their plasma membranes. Extracellular fluid (ECF) surrounds all cells in the body. Extracellular fluid has two primary constituents: the fluid component of the blood (called plasma) and the interstitial fluid (IF) that surrounds all cells not in the blood (Figure \(\PageIndex{2}\)).
Intracellular Fluid
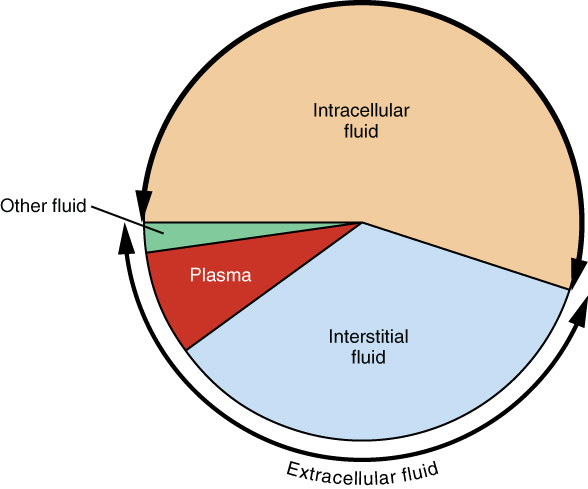
The ICF lies within cells and is the principal component of the cytosol/cytoplasm. The ICF makes up about 60 percent of the total water in the human body, and in an average-size adult male, the ICF accounts for about 25 liters (seven gallons) of fluid (Figure \(\PageIndex{3}\)). This fluid volume tends to be very stable, because the amount of water in living cells is closely regulated. If the amount of water inside a cell falls to a value that is too low, the cytosol becomes too concentrated with solutes to carry on normal cellular activities; if too much water enters a cell, the cell may burst and be destroyed.
Extracellular Fluid
The ECF accounts for the other one-third of the body’s water content. Approximately 20 percent of the ECF is found in plasma. Plasma travels through the body in blood vessels and transports a range of materials, including blood cells, proteins (including clotting factors and antibodies), electrolytes, nutrients, gases, and wastes. Gases, nutrients, and waste materials travel between capillaries and cells through the IF. Cells are separated from the IF by a selectively permeable cell membrane that helps regulate the passage of materials between the IF and the interior of the cell.
The body has other water-based ECF. These include the cerebrospinal fluid that bathes the brain and spinal cord, lymph, the synovial fluid in joints, the pleural fluid in the pleural cavities, the pericardial fluid in the cardiac sac, the peritoneal fluid in the peritoneal cavity, and the aqueous humor of the eye. Because these fluids are outside of cells, these fluids are also considered components of the ECF compartment.
Composition of Body Fluids
The compositions of the two components of the ECF—plasma and IF—are more similar to each other than either is to the ICF (Figure \(\PageIndex{4}\)). Blood plasma has high concentrations of sodium, chloride, bicarbonate, and protein. The IF has high concentrations of sodium, chloride, and bicarbonate, but a relatively lower concentration of protein. In contrast, the ICF has elevated amounts of potassium, phosphate, magnesium, and protein. Overall, the ICF contains high concentrations of potassium and phosphate (HPO42-), whereas both plasma and the ECF contain high concentrations of sodium and chloride.
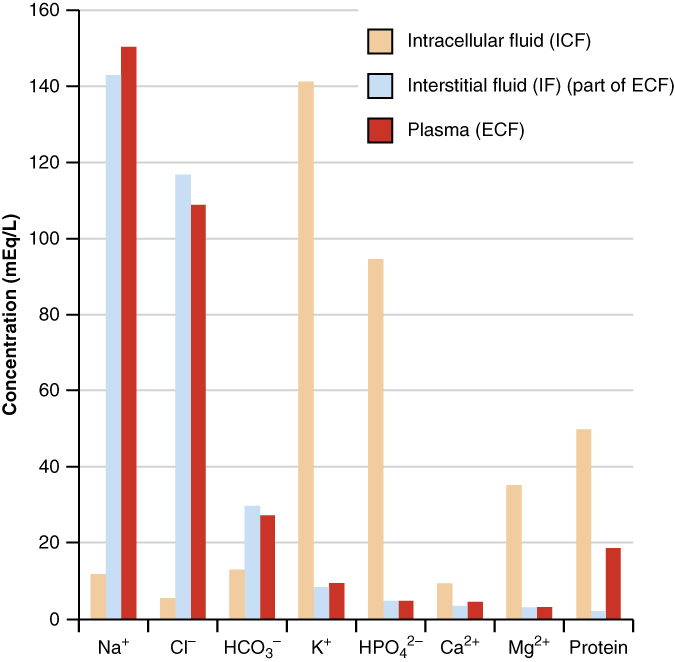
Most body fluids are neutral in charge. Thus, cations, or positively charged ions, and anions, or negatively charged ions, are balanced in fluids. As seen in the previous graph, sodium (Na+) ions and chloride (Cl-) ions are concentrated in the ECF of the body, whereas potassium (K+) ions are concentrated inside cells. Although sodium and potassium can “leak” through “pores” into and out of cells, respectively, the high levels of potassium and low levels of sodium in the ICF are maintained by sodium-potassium pumps in the cell membranes. These pumps use the energy supplied by ATP to pump sodium out of the cell and potassium into the cell (Figure \(\PageIndex{5}\)).
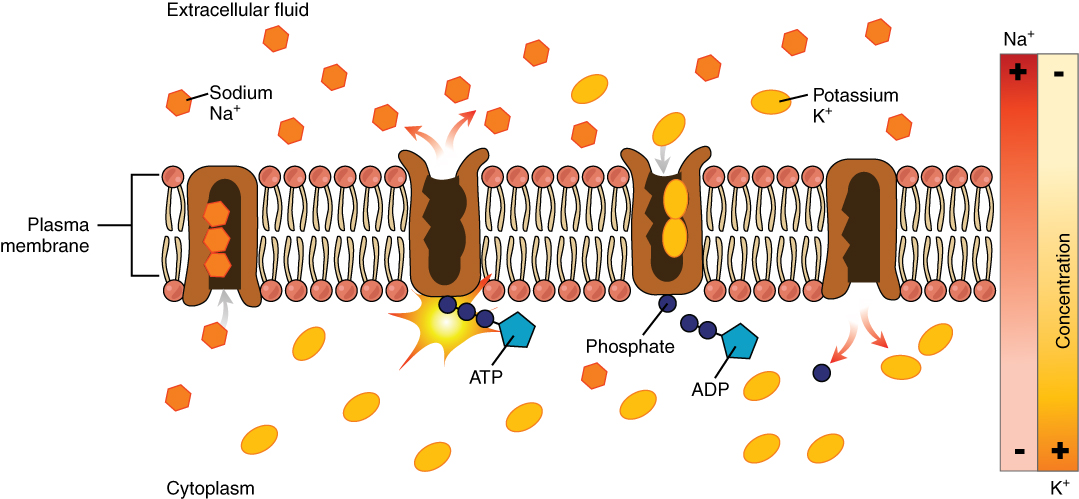
Fluid Movement between Compartments
Hydrostatic pressure, the force exerted by a fluid against a wall, causes movement of fluid between compartments. The hydrostatic pressure of blood is the pressure exerted by blood against the walls of the blood vessels by the pumping action of the heart. In capillaries, hydrostatic pressure (also known as capillary blood pressure) is higher than the opposing “colloid osmotic pressure” in blood—a “constant” pressure primarily produced by circulating albumin—at the arteriolar end of the capillary (Figure \(\PageIndex{6}\)). This pressure forces plasma and nutrients out of the capillaries and into surrounding tissues. Fluid and the cellular wastes in the tissues enter the capillaries at the venule end, where the hydrostatic pressure is less than the osmotic pressure in the vessel. Filtration pressure squeezes fluid from the plasma in the blood to the IF surrounding the tissue cells. The surplus fluid in the interstitial space that is not returned directly back to the capillaries is drained from tissues by the lymphatic system, and then re-enters the vascular system at the subclavian veins.
Disorders of Fluid Balance: Edema
Edema is the accumulation of excess water in the tissues. It is most common in the soft tissues of the extremities. The physiological causes of edema include water leakage from blood capillaries. Edema is almost always caused by an underlying medical condition, by the use of certain therapeutic drugs, by pregnancy, by localized injury, or by an allergic reaction. In the limbs, the symptoms of edema include swelling of the subcutaneous tissues, an increase in the normal size of the limb, and stretched, tight skin. One quick way to check for subcutaneous edema localized in a limb is to press a finger into the suspected area. Edema is likely if the depression persists for several seconds after the finger is removed (which is called “pitting”).
Pulmonary edema is excess fluid in the air sacs of the lungs, a common symptom of heart and/or kidney failure. People with pulmonary edema likely will experience difficulty breathing, and they may experience chest pain. Pulmonary edema can be life threatening, because it compromises gas exchange in the lungs, and anyone having symptoms should immediately seek medical care.
In pulmonary edema resulting from heart failure, excessive leakage of water occurs because fluids get “backed up” in the pulmonary capillaries of the lungs, when the left ventricle of the heart is unable to pump sufficient blood into the systemic circulation. Because the left side of the heart is unable to pump out its normal volume of blood, the blood in the pulmonary circulation gets “backed up,” starting with the left atrium, then into the pulmonary veins, and then into pulmonary capillaries. The resulting increased hydrostatic pressure within pulmonary capillaries, as blood is still coming in from the pulmonary arteries, causes fluid to be pushed out of them and into lung tissues.
Other causes of edema include damage to blood vessels and/or lymphatic vessels, or a decrease in osmotic pressure in chronic and severe liver disease, where the liver is unable to manufacture plasma proteins (Figure \(\PageIndex{8}\)). A decrease in the normal levels of plasma proteins results in a decrease of colloid osmotic pressure (which counterbalances the hydrostatic pressure) in the capillaries. This process causes loss of water from the blood to the surrounding tissues, resulting in edema.
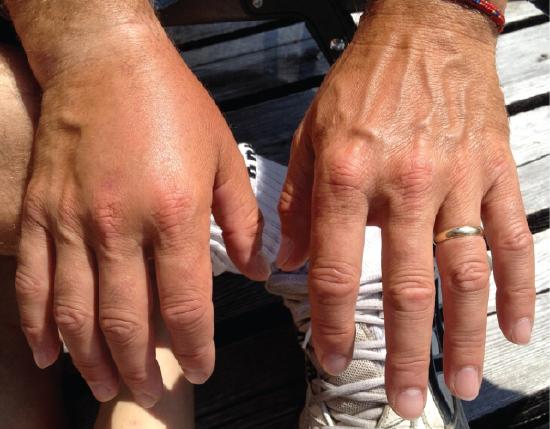
Figure \(\PageIndex{8}\): Edema. An allergic reaction can cause capillaries in the hand to leak excess fluid that accumulates in the tissues. (credit: Jane Whitney)
Mild, transient edema of the feet and legs may be caused by sitting or standing in the same position for long periods of time, as in the work of a toll collector or a supermarket cashier. This is because deep veins in the lower limbs rely on skeletal muscle contractions to push on the veins and thus “pump” blood back to the heart. Otherwise, the venous blood pools in the lower limbs and can leak into surrounding tissues.
Medications that can result in edema include vasodilators, calcium channel blockers used to treat hypertension, non-steroidal anti-inflammatory drugs, estrogen therapies, and some diabetes medications. Underlying medical conditions that can contribute to edema include congestive heart failure, kidney damage and kidney disease, disorders that affect the veins of the legs, and cirrhosis and other liver disorders.
Therapy for edema usually focuses on elimination of the cause. Activities that can reduce the effects of the condition include appropriate exercises to keep the blood and lymph flowing through the affected areas. Other therapies include elevation of the affected part to assist drainage, massage and compression of the areas to move the fluid out of the tissues, and decreased salt intake to decrease sodium and water retention.
Chapter Review
Your body is mostly water. Body fluids are aqueous solutions with differing concentrations of materials, called solutes. An appropriate balance of water and solute concentrations must be maintained to ensure cellular functions. If the cytosol becomes too concentrated due to water loss, cell functions deteriorate. If the cytosol becomes too dilute due to water intake by cells, cell membranes can be damaged, and the cell can burst. Hydrostatic pressure is the force exerted by a fluid against a wall and causes movement of fluid between compartments. Fluid can also move between compartments along an osmotic gradient. Active transport processes require ATP to move some solutes against their concentration gradients between compartments. Passive transport of a molecule or ion depends on its ability to pass easily through the membrane, as well as the existence of a high to low concentration gradient.
Essay Questions
Q. Read the text about body fluids, fluid compartments, and electrolytes. When blood volume decreases due to sweating, from what source is water taken in by the blood?
Answer: The interstitial fluid (IF).
Q. Watch this video to see an explanation of the dynamics of fluid in the body’s compartments. What happens in tissues when capillary blood pressure is less than osmotic pressure?
Answer: Fluid enters the capillaries from interstitial spaces.
Review Questions
Q. Solute contributes to the movement of water between cells and the surrounding medium by ? .
A. osmotic pressure
B. hydrostatic pressure
C. Brownian movement
D. random motion
- Answer:
- A
Q. A cation has a(n) ? charge.
A. neutral
B. positive
C. alternating
D. negative
- Answer:
- B
Q. Interstitial fluid (IF) is ? .
A. the fluid in the cytosol of the cells
B. the fluid component of blood
C. the fluid that bathes all of the body’s cells except for blood cells
D. the intracellular fluids found between membranes
- Answer:
- C
Critical Thinking Questions
Q. Plasma contains more sodium than chloride. How can this be if individual ions of sodium and chloride exactly balance each other out, and plasma is electrically neutral?
A. There are additional negatively charged molecules in plasma besides chloride. The additional sodium balances the total negative charges.
Q. How is fluid moved from compartment to compartment?
A. Fluid is moved by a combination of osmotic and hydrostatic pressures. The osmotic pressure results from differences in solute concentrations across cell membranes. Hydrostatic pressure results from the pressure of blood as it enters a capillary system, forcing some fluid out of the vessel into the surrounding tissues.
Glossary
- extracellular fluid (ECF)
- fluid exterior to cells; includes the interstitial fluid, blood plasma, and fluids found in other reservoirs in the body
- fluid compartment
- fluid inside all cells of the body constitutes a compartment system that is largely segregated from other systems
- hydrostatic pressure
- pressure exerted by a fluid against a wall, caused by its own weight or pumping force
- interstitial fluid (IF)
- fluid in the small spaces between cells not contained within blood vessels
- intracellular fluid (ICF)
- fluid in the cytosol of cells
Contributors and Attributions
OpenStax Anatomy & Physiology (CC BY 4.0). Access for free at https://openstax.org/books/anatomy-and-physiology


