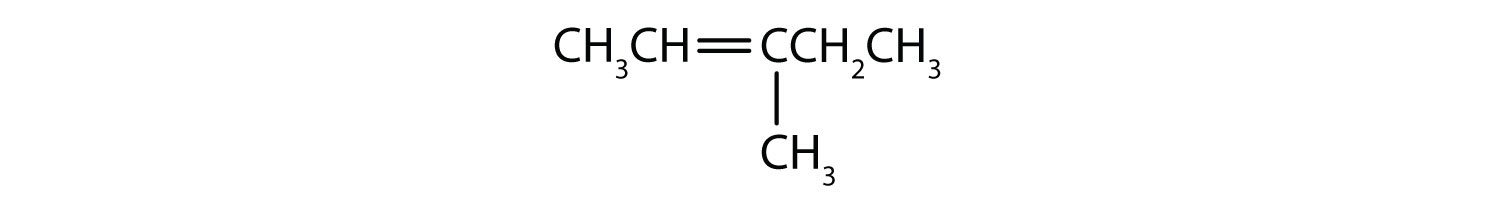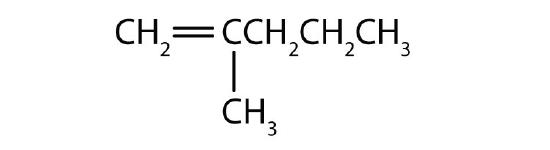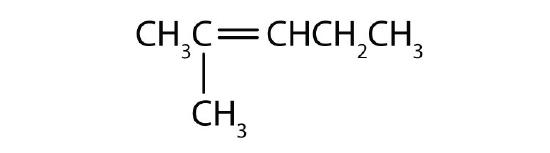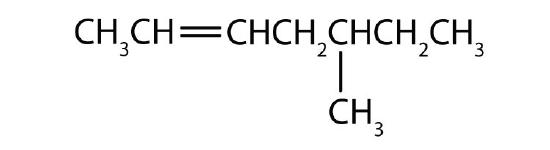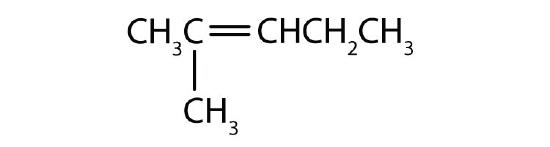6.E: Unsaturated and Aromatic Hydrocarbons (Exercises)
- Page ID
- 60603
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)13.1: Alkenes- Structures and Names
Concept Review Exercises
- Briefly identify the important distinctions between a saturated hydrocarbon and an unsaturated hydrocarbon.
- Briefly identify the important distinctions between an alkene and an alkane.
- Classify each compound as saturated or unsaturated. Identify each as an alkane, an alkene, or an alkyne.
-
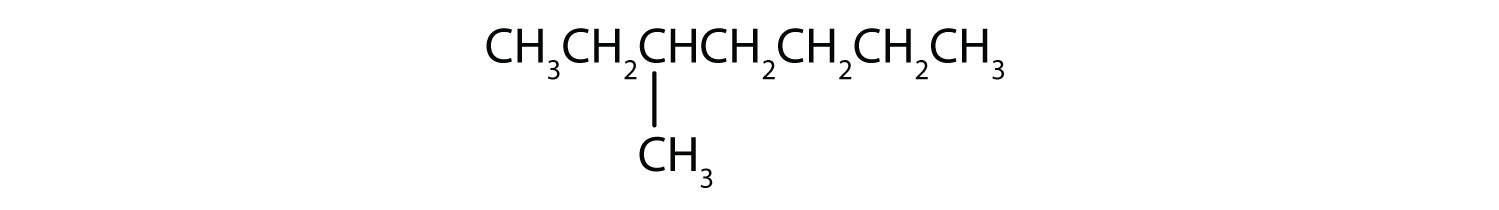
- CH3CH2C≡CCH3
-
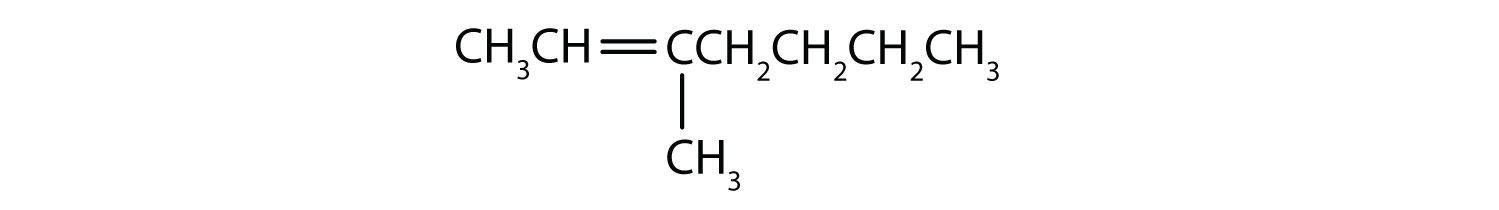
-
Answers
- Unsaturated hydrocarbons have double or triple bonds and are quite reactive; saturated hydrocarbons have only single bonds and are rather unreactive.
- An alkene has a double bond; an alkane has single bonds only.
-
- saturated; alkane
- unsaturated; alkyne
- unsaturated; alkene
Exercises
- Draw the structure for each compound.
- 2-methyl-2-pentene
- 2,3-dimethyl-1-butene
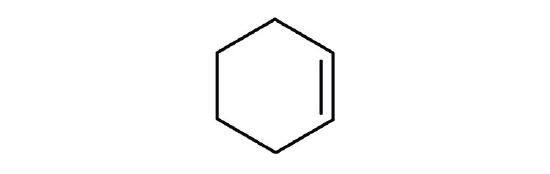
- cyclohexene
- Draw the structure for each compound.
- 5-methyl-1-hexene
- 3-ethyl-2-pentene
- 4-methyl-2-hexene
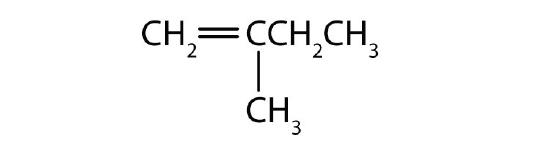
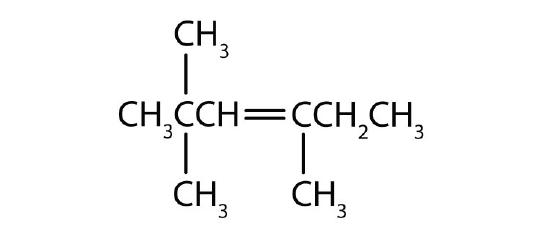
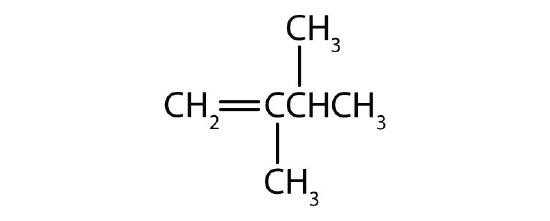
- Name each compound according to the IUPAC system.
-
- Name each compound according to the IUPAC system.
-
Answers
-
- 2-methyl-1-pentene
- 2-methyl-2-pentene
- 2,5-dimethyl-2-hexene
13.2: Cis-Trans Isomers (Geometric Isomers)
Concept Review Exercises
- What are cis-trans (geometric) isomers? What two types of compounds can exhibit cis-trans isomerism?
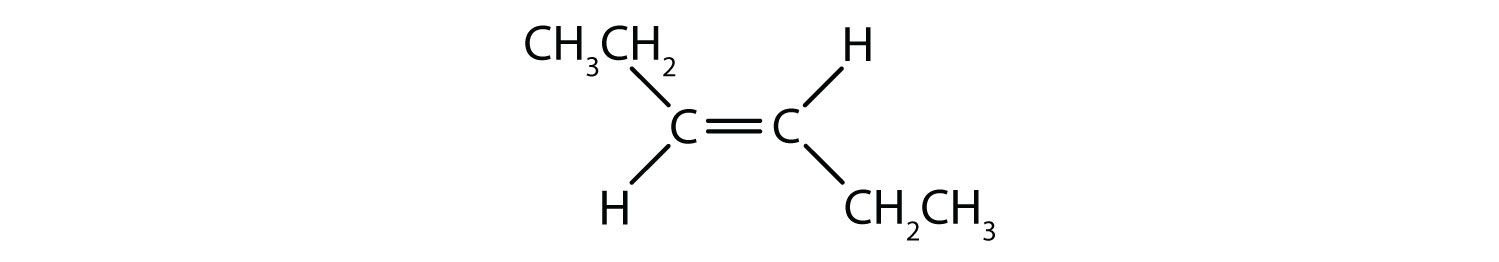
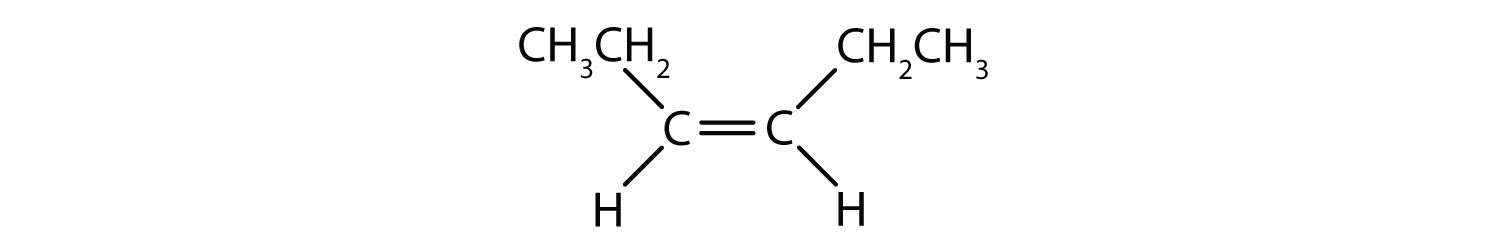
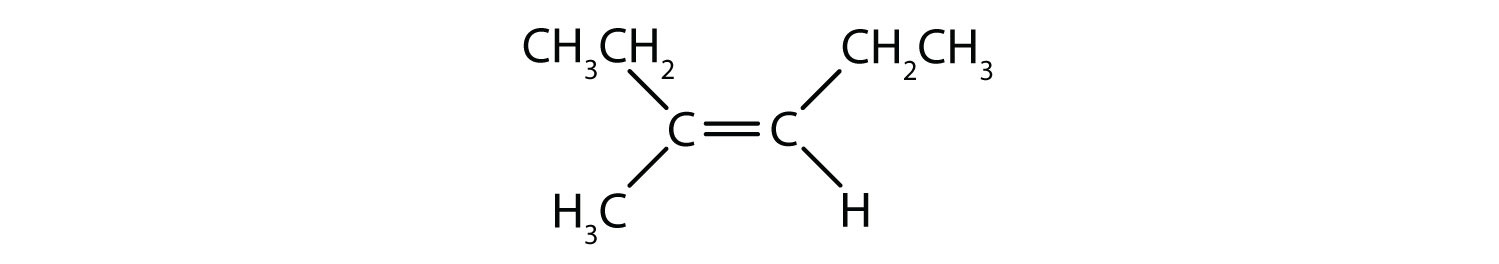
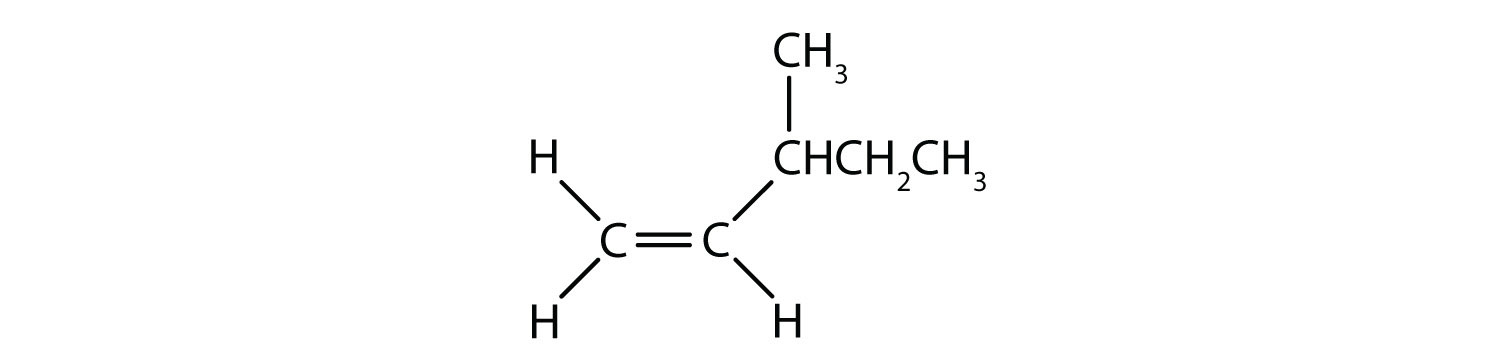
- Classify each compound as a cis isomer, a trans isomer, or neither.
-
Answers
- Cis-trans isomers are compounds that have different configurations (groups permanently in different places in space) because of the presence of a rigid structure in their molecule. Alkenes and cyclic compounds can exhibit cis-trans isomerism.
-
- trans (the two hydrogen atoms are on opposite sides)
- cis (the two hydrogen atoms are on the same side, as are the two ethyl groups)
- cis (the two ethyl groups are on the same side)
- neither (flipping the bond does not change the molecule. There are no isomers for this molecule)
Exercises
- Draw the structures of the cis-trans isomers for each compound. Label them cis and trans. If no cis-trans isomers exist, write none.
- 2-bromo-2-pentene
- 3-hexene
- 4-methyl-2-pentene
- 1,1-dibromo-1-butene
- 2-butenoic acid (CH3CH=CHCOOH)
- Draw the structures of the cis-trans isomers for each compound. Label them cis and trans. If no cis-trans isomers exist, write none.
- 2,3-dimethyl-2-pentene
- 1,1-dimethyl-2-ethylcyclopropane
- 1,2-dimethylcyclohexane
- 5-methyl-2-hexene
- 1,2,3-trimethylcyclopropane
Answer
-
a: none. There are two distinct geometric isomers, but since there are there are four different groups off the double bond, these are both cis/trans isomers (they are technically E/Z isomers discussed elsewhere).b:

c:
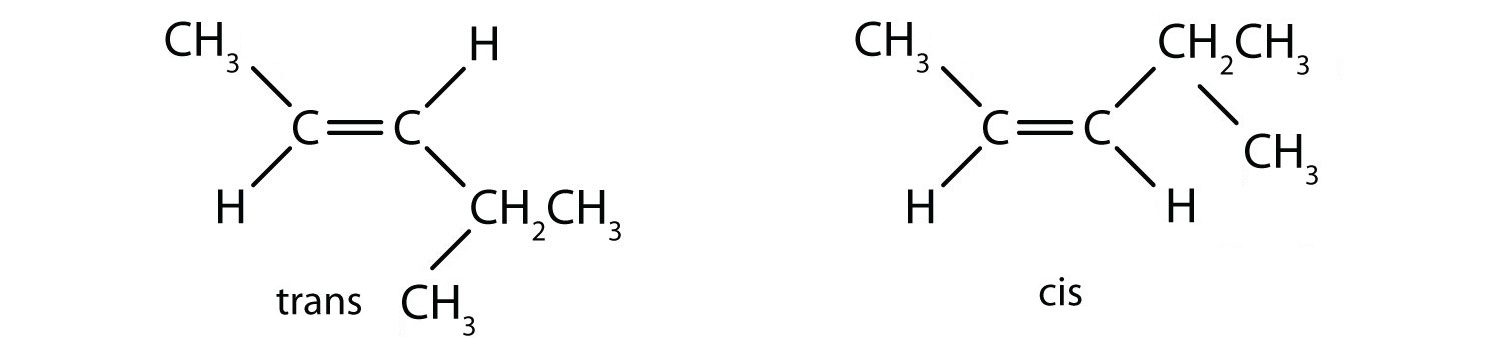
d:
 e:
e: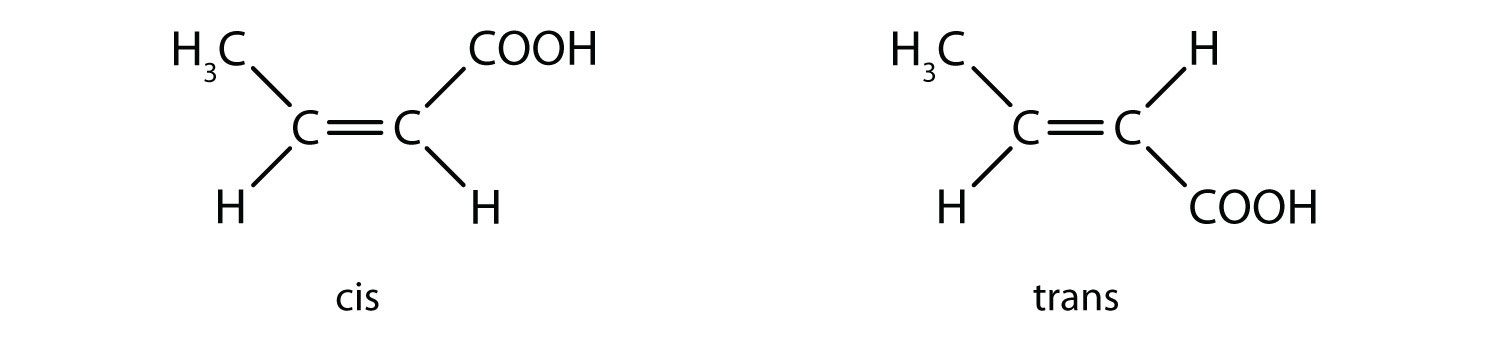
13.3: Physical Properties of Alkenes
Concept Review Exercises
- Briefly describe the physical properties of alkenes. How do these properties compare to those of the alkanes?
- Without consulting tables, arrange the following alkenes in order of increasing boiling point: 1-butene, ethene, 1-hexene, and propene.
Answers
- Alkenes have physical properties (low boiling points, insoluble in water) quite similar to those of their corresponding alkanes.
- ethene < propene < 1-butene < 1-hexene
Exercises
- Without referring to a table or other reference, predict which member of each pair has the higher boiling point.
- 1-pentene or 1-butene
- 3-heptene or 3-nonene
- Which is a good solvent for cyclohexene, pentane or water?
Answer
-
- 1-pentene
- 3-nonene
13.4: Chemical Properties of Alkenes
Concept Review Exercises
- What is the principal difference in properties between alkenes and alkanes? How are they alike?
- If C12H24 reacts with HBr in an addition reaction, what is the molecular formula of the product?
Answers
- Alkenes undergo addition reactions; alkanes do not. Both burn.
- C12H24Br2
Exercises
- Complete each equation.
- (CH3) 2C=CH2 + Br2 →
- \(\mathrm{CH_2\textrm{=C}(CH_3)CH_2CH_3 + H_2 \xrightarrow{Ni}}\)
-

- Complete each equation.
- \(\mathrm{CH_2\textrm{=CHCH=C}H_2 + 2H_2\xrightarrow{Ni}}\)
- \(\mathrm{(CH_3)_2\textrm{C=C}(CH_3)_2 + H_2O \xrightarrow{H_2SO_4}}\)
-
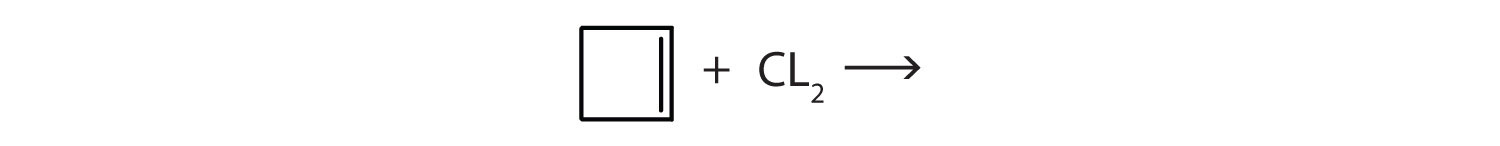
Answer
-
- (CH3)2CBrCH2Br
- CH3CH(CH3)CH2CH3
-
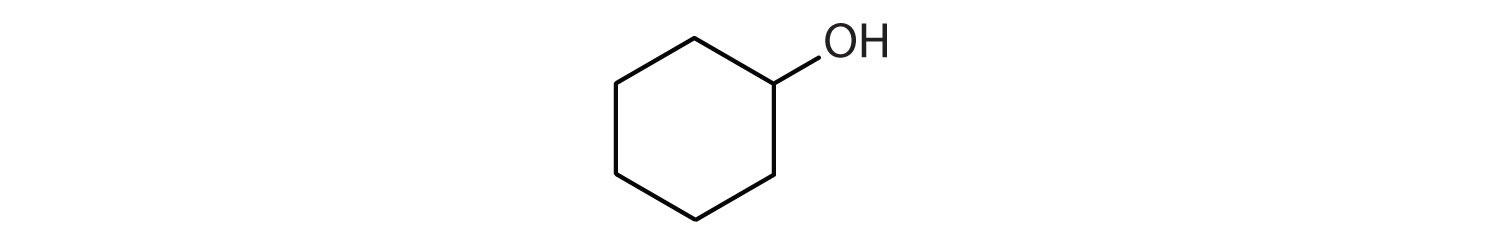
13.5: Polymers
Concept Review Exercises
- What is a monomer? What is a polymer? How do polymer molecules differ from the molecules we have discussed in earlier sections of this chapter?
- What is addition polymerization? What structural feature usually characterizes molecules used as monomers in addition polymerization?
- What is the molecular formula of a polymer molecule formed by the addition polymerization of 175 molecules of vinyl chloride (CH2=CHCl)?
Answers
- Monomers are small molecules that can be assembled into giant molecules referred to as polymers, which are much larger than the molecules we discussed earlier in this chapter.
- In addition polymerization, the monomers add to one another in such a way that the polymer contains all the atoms of the starting monomers.
- C350H525Cl175
Exercises
-
Write the condensed structural formula of the monomer from which Saran is formed. A segment of the Saran molecule has the following structure: CH2CCl2CH2CCl2CH2CCl2CH2CCl2.
-
Write the condensed structural formula for the section of a molecule formed from four units of the monomer CH2=CHF.
Answer
-
H2C=CCl2
13.6: Alkynes
Concept Review Exercises
- Briefly identify the important differences between an alkene and an alkyne. How are they similar?
- The alkene (CH3)2CHCH2CH=CH2 is named 4-methyl-1-pentene. What is the name of (CH3)2CHCH2C≡CH?
- Do alkynes show cis-trans isomerism? Explain.
Answers
- Alkenes have double bonds; alkynes have triple bonds. Both undergo addition reactions.
- 4-methyl-1-pentyne
- No; a triply bonded carbon atom can form only one other bond. It would have to have two groups attached to show cis-trans isomerism.
Exercises
- Draw the structure for each compound.
- acetylene
- 3-methyl-1-hexyne
- Draw the structure for each compound.
- 4-methyl-2-hexyne
- 3-octyne
- Name each alkyne.
- CH3CH2CH2C≡CH
- CH3CH2CH2C≡CCH3
Answers
-
- H–C≡C–H
-
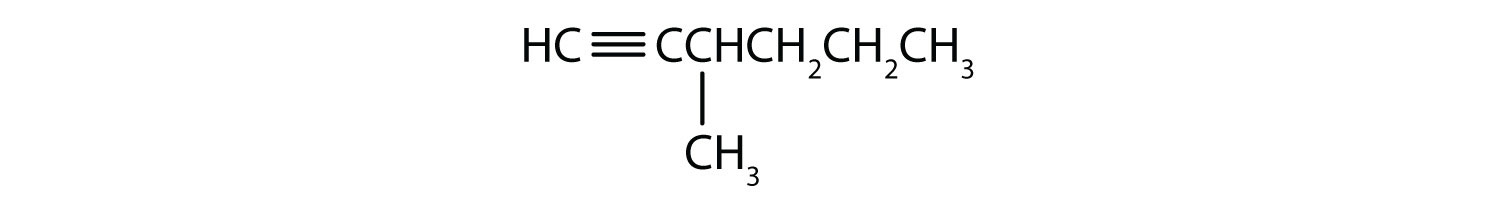
-
- 1-pentyne
- 2-hexyne
13.7: Aromatic Compounds- Benzene
Concept Review Exercises
- How do the typical reactions of benzene differ from those of the alkenes?
- Briefly describe the bonding in benzene.
- What does the circle mean in the chemist’s representation of benzene?
Answers
- Benzene is rather unreactive toward addition reactions compared to an alkene.
- Valence electrons are shared equally by all six carbon atoms (that is, the electrons are delocalized).
- The six electrons are shared equally by all six carbon atoms.
Exercises
- Draw the structure of benzene as if it had alternate single and double bonds.
- Draw the structure of benzene as chemists usually represent it today.
Answer
13.8: Structure and Nomenclature of Aromatic Compounds
Concept Review Exercises
- Briefly identify the important characteristics of an aromatic compound.
- What is meant by the prefixes meta, ortho, or para? Give the name and draw the structure for a compound that illustrates each.
- What is a phenyl group? Give the structure for 3-phenyloctane.
Answers
- An aromatic compound is any compound that contains a benzene ring or has certain benzene-like properties.
- meta = 1,3 disubstitution; (answers will vary)
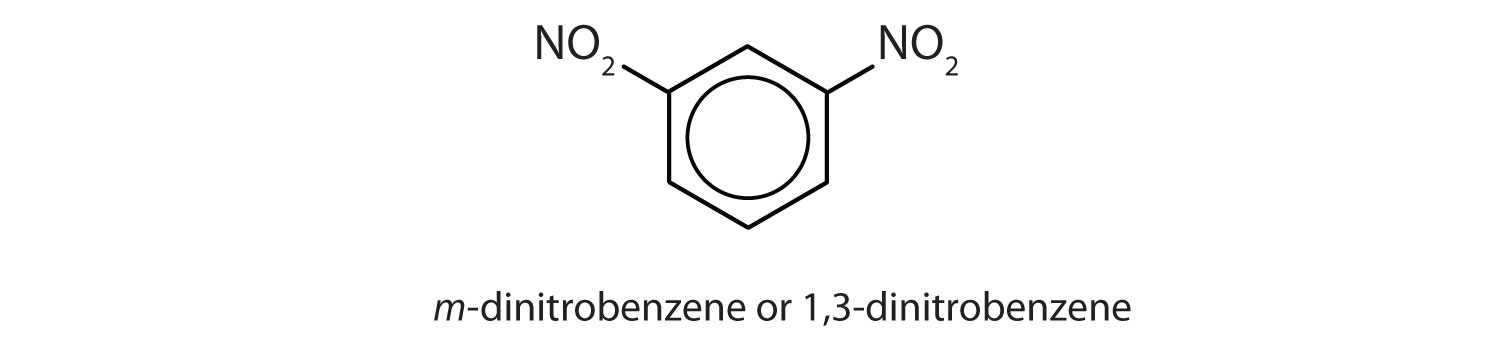
ortho = 1,2 disubstitution
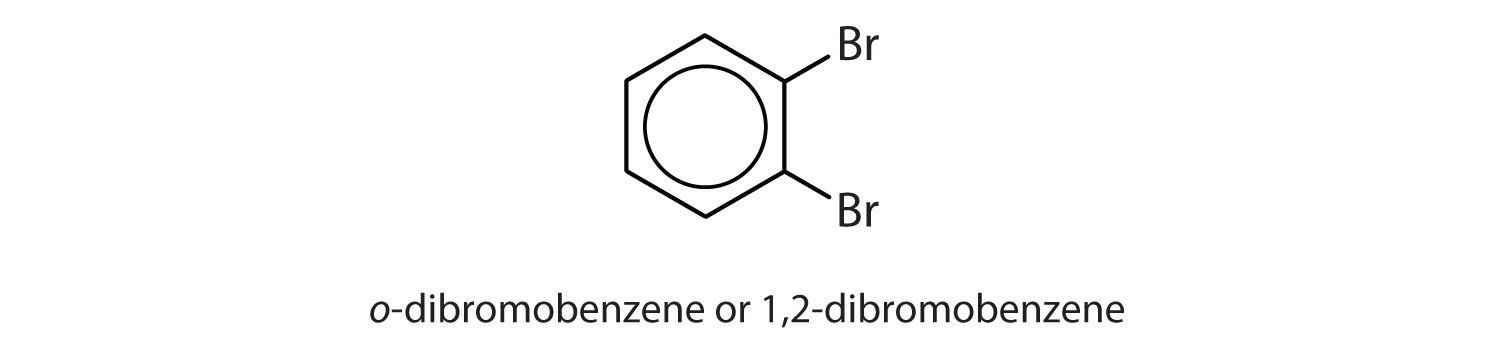
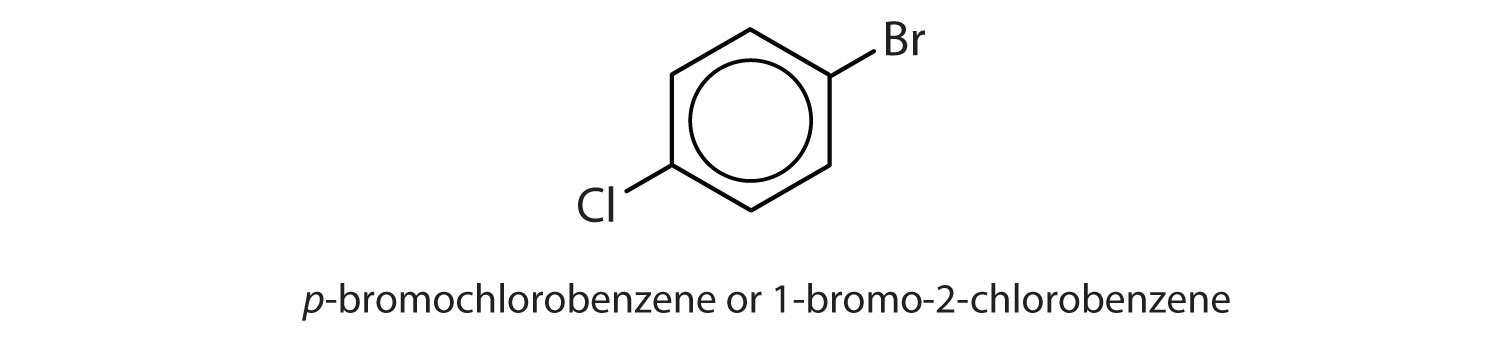
para = 1,4 disubstitution or 1-bromo-4-chlorobenzene
- phenyl group: C6H5 or
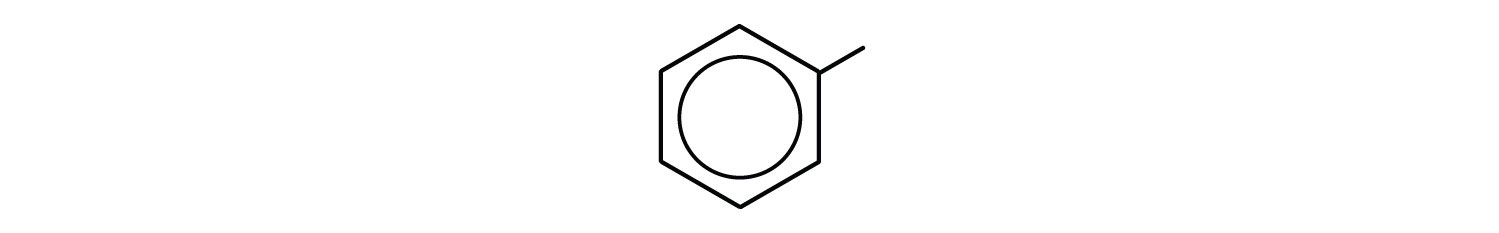
3-phenyloctane:
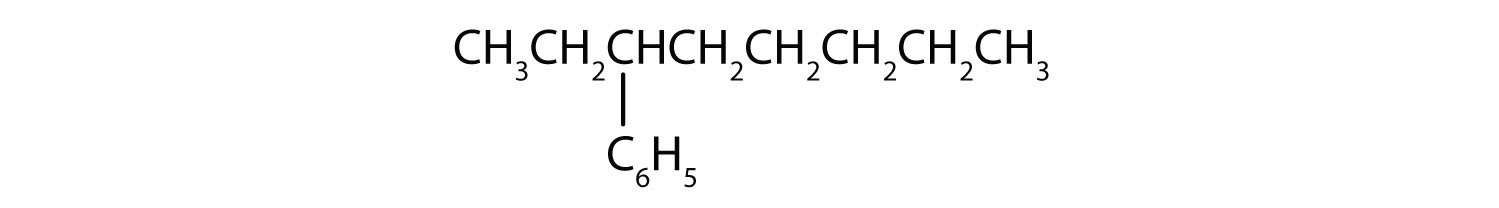
Exercises
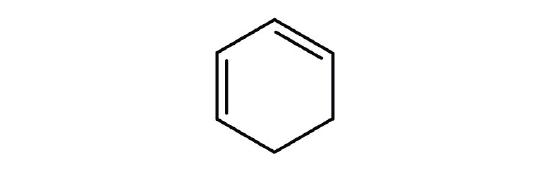
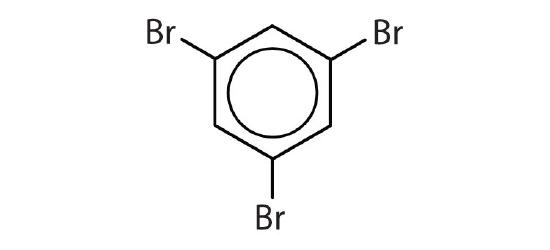
- Is each compound aromatic?
-
- Is each compound aromatic?
-
- Draw the structure for each compound.
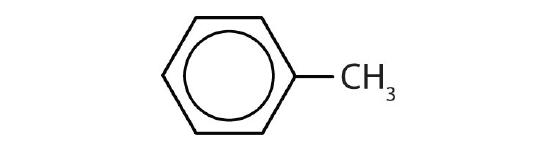
- toluene
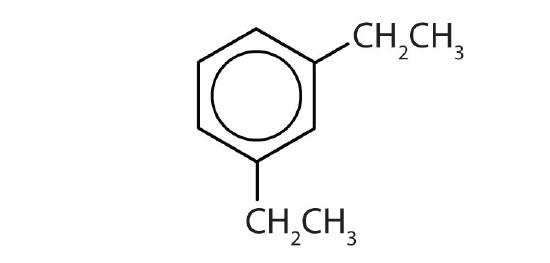
- m-diethylbenzene
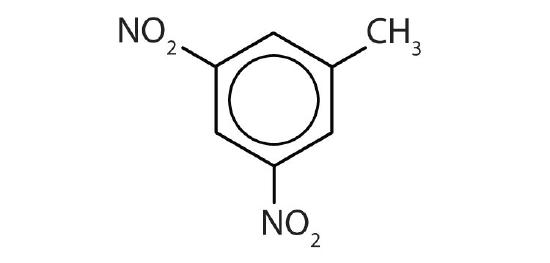
- 3,5-dinitrotoluene
- Draw the structure for each compound.
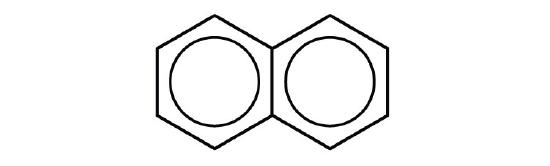
- p-dichlorobenzene
- naphthalene
- 1,2,4-trimethylbenzene
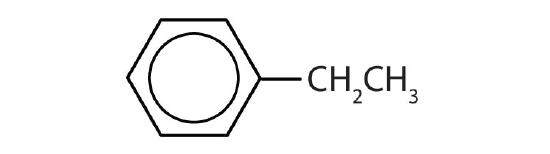
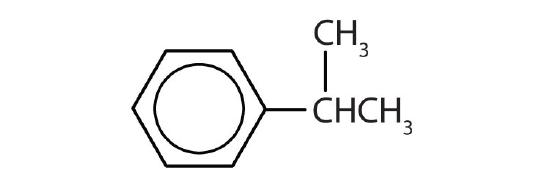
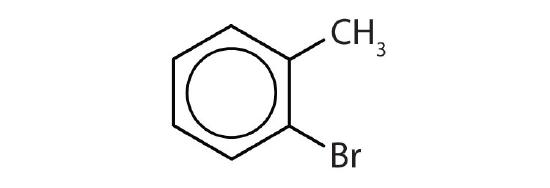
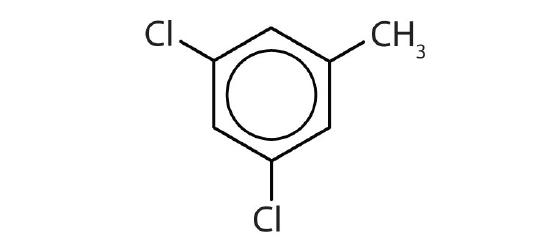
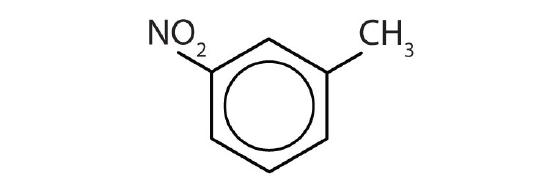
- Name each compound with its IUPAC name.
-
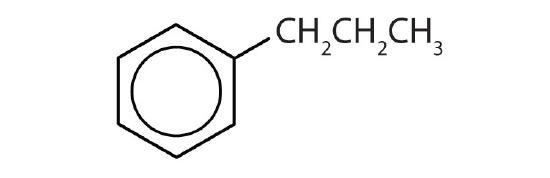
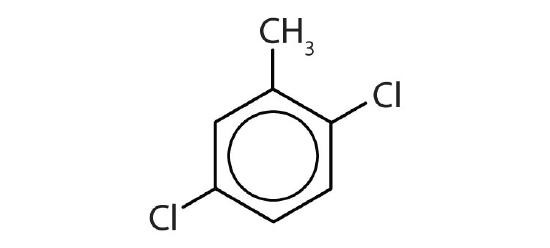
- Name each compound with its IUPAC name.
-
Answers
-
- yes
- no
-
- ethylbenzene
- isopropylbenzene
- o-bromotoluene
- 3,5-dichlorotoluene
Additional Exercises
-
Classify each compound as saturated or unsaturated.
-
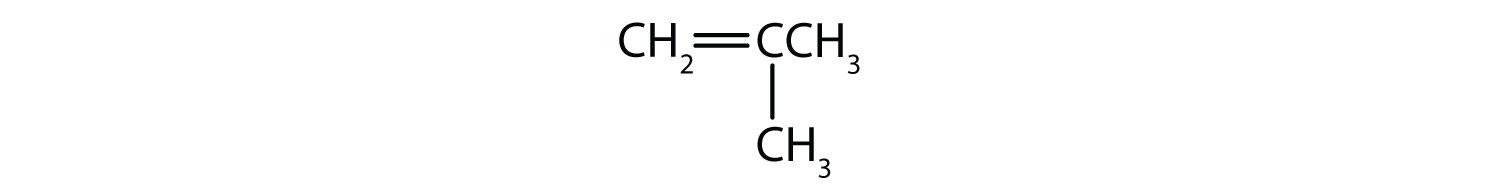
- CH3C≡CCH3
-
-
Classify each compound as saturated or unsaturated.
-
-
Give the molecular formula for each compound.
-
-
When three isomeric pentenes—X, Y, and Z—are hydrogenated, all three form 2-methylbutane. The addition of Cl2 to Y gives 1,2-dichloro-3-methylbutane, and the addition of Cl2 to Z gives 1,2-dichloro-2-methylbutane. Draw the original structures for X, Y, and Z.
-
Pentane and 1-pentene are both colorless, low-boiling liquids. Describe a simple test that distinguishes the two compounds. Indicate what you would observe.
-
Draw and name all the alkene cis-trans isomers corresponding to the molecular formula C5H10. (Hint: there are only two.)
-
The complete combustion of benzene forms carbon dioxide and water:
C6H6 + O2 → CO2 + H2OBalance the equation. What mass, in grams, of carbon dioxide is formed by the complete combustion of 39.0 g of benzene?
-
Describe a physiological effect of some PAHs.
-
What are some of the hazards associated with the use of benzene?
-
What is wrong with each name? Draw the structure and give the correct name for each compound.
- 2-methyl-4-heptene
- 2-ethyl-2-hexene
- 2,2-dimethyl-3-pentene
-
What is wrong with each name?
- 2-bromobenzene
- 3,3-dichlorotoluene
- 1,4-dimethylnitrobenzene
-
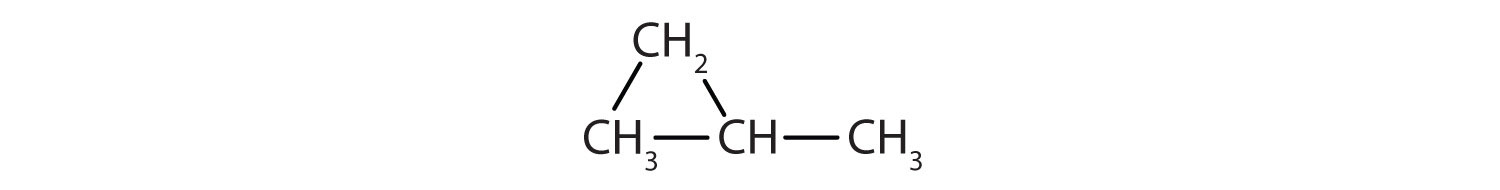
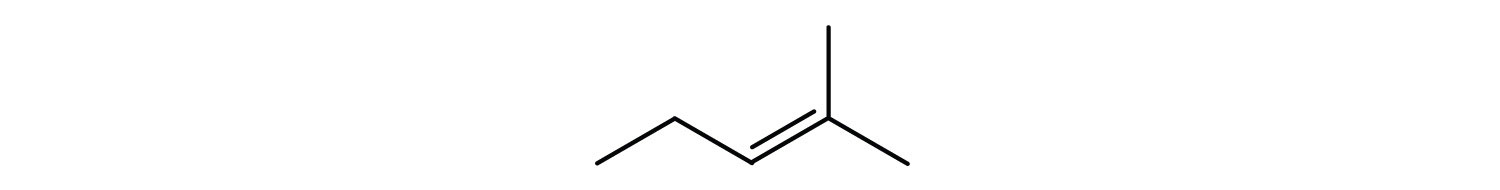
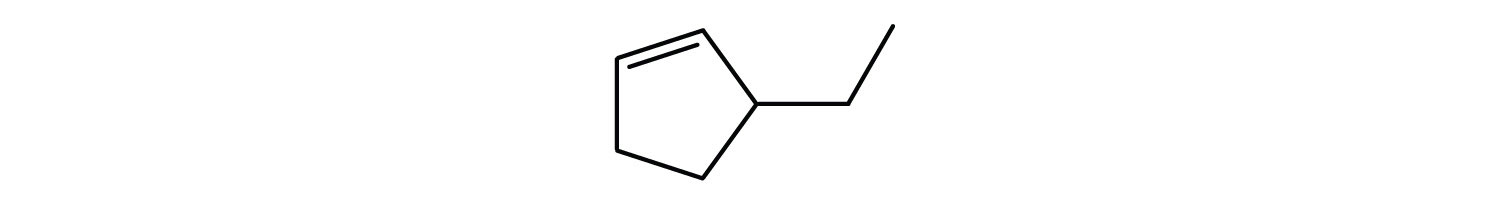
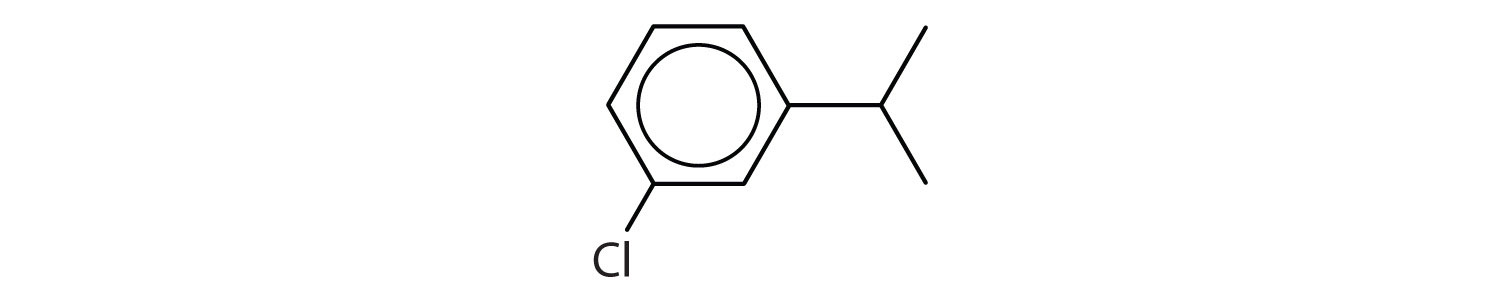
Following are line-angle formulas for three compounds. Draw the structure and give the name for each.
-
-
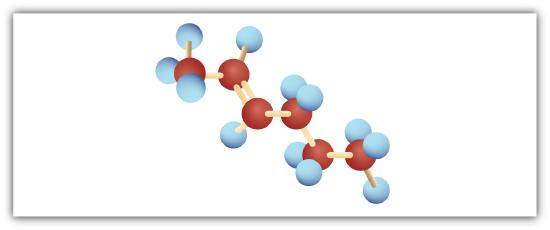
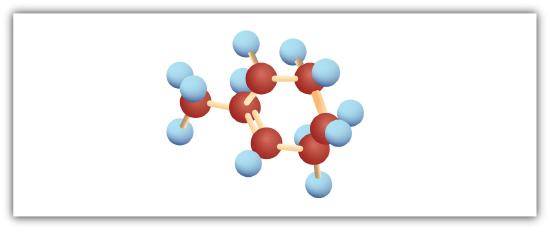
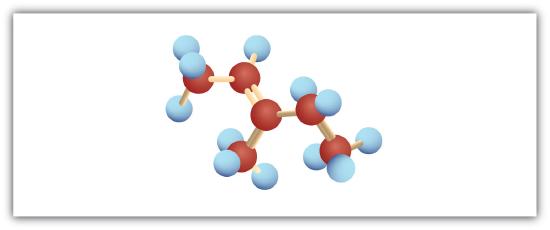
Following are ball-and-stick molecular models for three compounds (blue balls represent H atoms; red balls are C atoms). Write the condensed structural formula and give the name for each.
-
Answers
-
- unsaturated
- unsaturated
-
- C6H10
- C4H8
-
Add bromine solution (reddish-brown) to each. Pentane will not react, and the reddish-brown color persists; 1-pentene will react, leaving a colorless solution.
-
2C6H6 + 15O2 → 12CO2 + 6H2O; 132 g
-
carcinogenic, flammable
-
- number not needed
- can’t have two groups on one carbon atom on a benzene ring
- can’t have a substituent on the same carbon atom as the nitro group
-
- CH3CH=CHCH2CH2CH3; 2-hexene
-
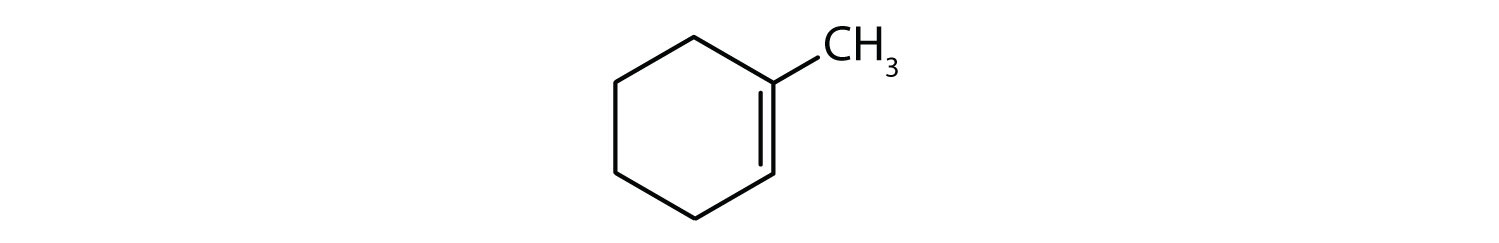
-