5.10: Surgical Reanimation Techniques for Facial Palsy/Paralysis
- Page ID
- 53274
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)OPEN ACCESS ATLAS OF OTOLARYNGOLOGY, HEAD & NECK OPERATIVE SURGERY
SURGICAL REANIMATION TECHNIQUES FOR FACIAL PALSY/PARALYSIS
David Sainsbury, Gregory Borschel, Ronald Zuker
The facial nerve not only provides conscious and subconscious motor control of the facial musculature, but also enables ocular protection, nasal airflow, oral continence and bilabial speech articulation. Additionally, it facilitates a defining human characteristic, the ability to smile. Consequently, facial nerve injury may inflict a spectrum of functional, aesthetic and psychosocial insults. Facial disfigurement has been described as the “last bastion of discrimination” and those with facial palsy may be considered “different”, suffering social and workplace discrimination (McGrouther 1997). This may lead to social isolation, decreased self-esteem and negative self-image (Newell 1991) and there is a high incidence of depression in individuals with facial palsy (Ryzenman et al. 2005). As with any form of disfigurement the degree of injury does not necessarily correlate with the severity of psychosocial disturbance (Robinson 1997). Moreover, psychological stress rather than functional deficit, is often the key determinant in predicting social handicap and need for surgical intervention (Van Swearingen et al. 1998; Bradbury et al. 2006).
Whenever possible, surgeons should promptly repair facial nerve injuries in order to restore neural continuity from the facial motor nucleus to the distal facial nerve branches. Such repairs may involve direct microsurgical repair or nerve grafts. If these are not possible then other techniques may be used to re-establish facial resting symmetry and mimetic movement. This chapter explores the anatomy and etiology of facial nerve injury and then focuses on the management of facial palsy with particular emphasis on facial reanimation.
Anatomy
The facial nerve (cranial nerve VII) is embryologically derived from, and consequently innervates all structures originating from, the second branchial arch. Arbitrarily, the facial nerve can be divided into three segments along its course: intracranial, intratemporal and extratemporal.
1. Intracranial anatomy
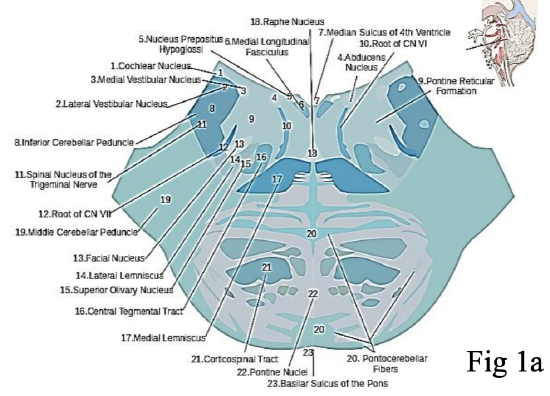
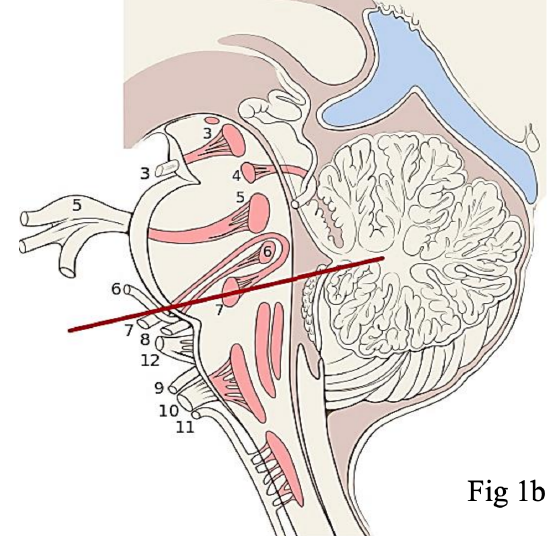
Figures 1a,b: Origin of the facial nerve from the pontine region of the brainstem. Note the close proximity of the facial nerve (cranial nerve VII) and vestibulocochlear (cranial nerve VIII) at the cerebellar pontine angle. This explains why vestibular schwannomas, or surgery to remove them, may result in facial nerve injury
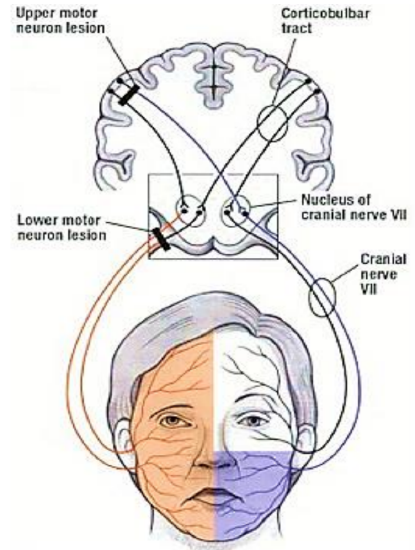
Figure 2: The cell bodies giving rise to the frontal branch of the facial nerve receive bilateral cortical input explaining why an upper motor neurone lesion results in contralateral facial paralysis with sparing of the frontalis muscle. Conversely, a lower motor neurone lesion to the facial nerve causes ipsilateral facial hemiplegia, including the ipsilateral frontalis mus cle (http://teachmeanatomy.info/neuro/des...g-tracts-motor)
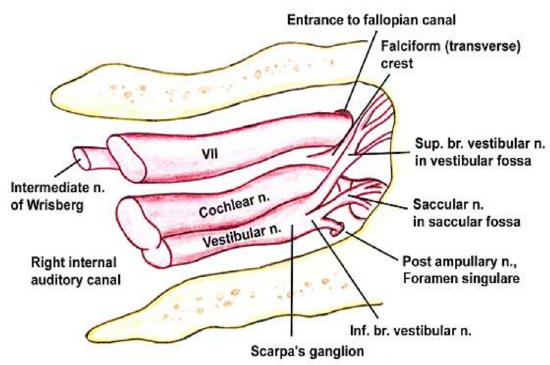
Figure 3: The facial nerve enters the internal auditory canal of the temporal bone with the vestibulocochlear nerve (VIII cranial n) and the nervus intermedius (Nerve of Wrisberg). The nervus intermedius is part of the facial nerve between the motor component of the facial nerve and the vestibulocochlear nerve. It transmits sensory and parasympathetic fibers http://emedicine.medscape.com/articl...view#aw2aab6b5
The facial nerve originates from the pontine region of the brain stem (Figure 1). The cell bodies giving rise to the frontal branch receive bilateral cortical input (all the other facial nuclei cell bodies receive contralateral cortical input). This explains why an ipsilateral supranuclear lesion (upper motor neurone lesion) to the facial nerve results in contralateral facial paralysis but with bilateral sparing of the frontalis muscle. Conversely, a lower motor neurone lesion to the facial nerve results in ipsilateral facial hemiplegia, including the ipsilateral frontalis (Figure 2). The facial nerve enters the temporal bone at the internal auditory meatus with the eighth cranial nerve, the vestibulocochlear nerve (Figure 3).
2. Intratemporal anatomy (Figures 4, 5)
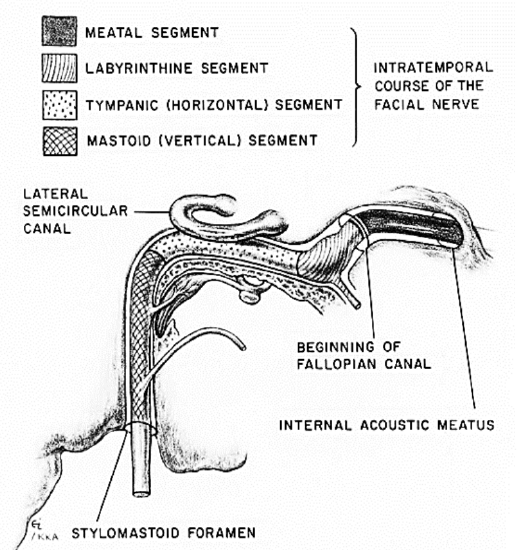
Figure 4: Intratemporal course of VII nerve
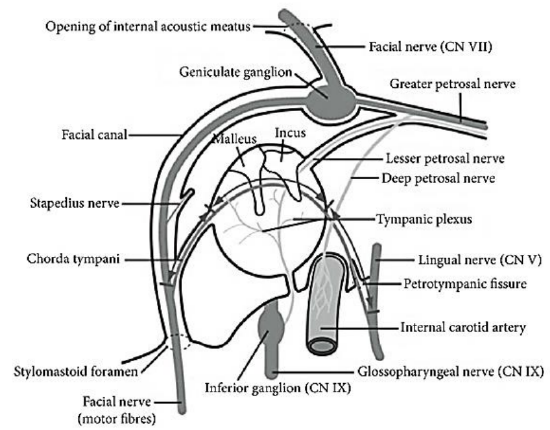
Figure 5: The intratemporal course of the VII nerve from internal auditory meatus to stylomastoid foramen, with its branches
The facial nerve enters the internal auditory canal and travels with the acoustic and vestibular nerves for about 8-10 mm. It then enters the Fallopian canal, which has 3 segments:
a. Labyrinthine segment: This is 3-5 mm long, and courses from the entrance of fallopian canal to the geniculate ganglion. It is the narrowest segment of the fallopian canal, with a mean diameter of 1.42 mm and with the nerve occupying 83% of available space. At the geniculate ganglion the first branch of the facial nerve, the greater petrosal nerve, is given off. The greater petrosal nerve provides parasympathetic innervation to the lacrimal and palatal glands. The junction of labyrinthine and tympanic segments forms an acute angle and traumatic facial nerve shearing may occur here.
b. Tympanic segment: This is 8-11 mm long, and courses from the geniculate ganglion to a bend at the lateral semicircular canal.
c. Mastoid segment: This is 9-12 mm long and extends from the angle at the lateral semicircular canal to stylomastoid foramen. This segment has the widest cross-sectional diameter and gives off three branches:
- Tympanic nerve: This is a small sensory branch to the external auditory canal. Injury may cause hyperesthesia of part of the external auditory canal, known as Hitselberger’s sign
- Nerve to stapedius: The stapedius muscle dampens loud noise. Interestingly, the cell bodies of the motor nerve are not located in the facial nucleus and consequently the stapedius muscle is unaffected in Möbius syndrome
- Chorda tympani: This is the last intratemporal branch of the facial nerve; it joins the lingual nerve to provide parasympathetic innervation to submandibular and sublingual glands and taste sensation to anterior two-thirds of the tongue
- Nerve to stapedius: The stapedius muscle dampens loud noise. Interestingly, the cell bodies of the motor nerve are not located in the facial nucleus and consequently the stapedius muscle is unaffected in Möbius syndrome
- Chorda tympani: This is the last intratemporal branch of the facial nerve; it joins the lingual nerve to provide parasympathetic innervation to submandibular and sublingual glands and taste sensation to anterior two-thirds of the tongue
3. Extratemporal anatomy
The facial nerve exits the temporal bone at the stylomastoid foramen. Here the mastoid tip, the tympanic ring and mandibular ramus confer protection. However, in children under 2 years of age the nerve is superficial and more prone to injury.
The posterior auricular nerve is the first branch of the facial nerve following its departure from the stylomastoid foramen and innervates the superior and posterior auricular muscles, the occipital muscles and provides sensory innervation to a small area behind the earlobe.
The next branch is the motor branch to the posterior belly of digastric and stylohyoid muscles. Here the facial nerve is located just above the posterior belly of digastric.
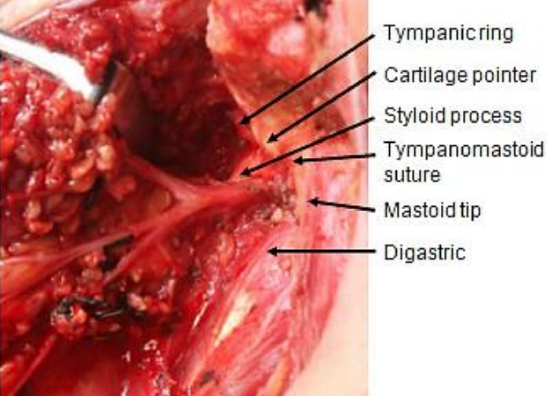
Figure 6: Intraoperative surgical landmarks for the facial nerve trunk
Anatomical pointers to find the trunk of the facial nerve where it exits the stylomastoid foramen include the posterior belly of digastric, tympanomastoid suture line, tragal pointer and styloid process (Figure 6). These are described in detail in the Parotidectomy chapter.
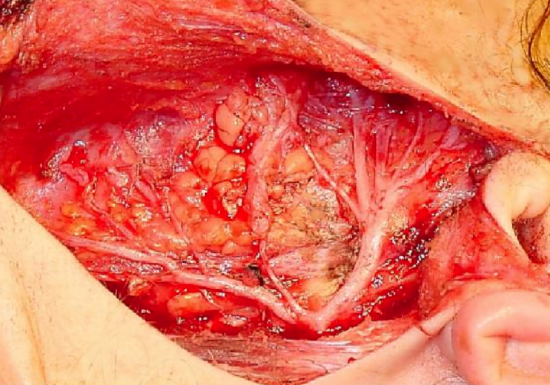
Figure 7: The superficial lobe of the parotid has been removed to expose the facial nerve trunk dividing into superior and inferior divisions at the pes anserinus
The facial nerve then enters the parotid gland and arborizes between the deep and superficial lobes of the parotid gland. The nerve first divides into temporozygomatic and cervicofacial divisions (Figures 6, 7).
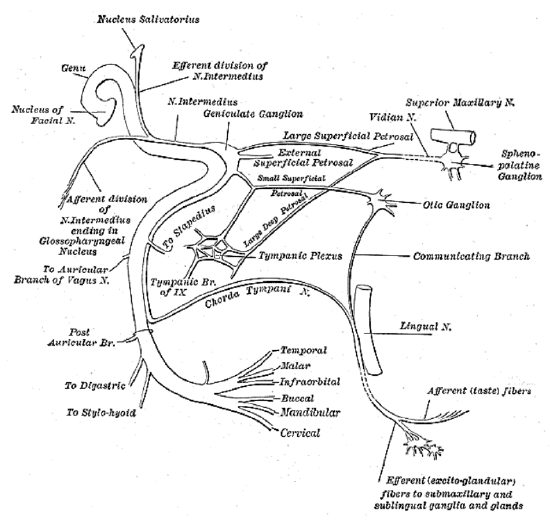
Figure 8: Extratemporal course of facial nerve
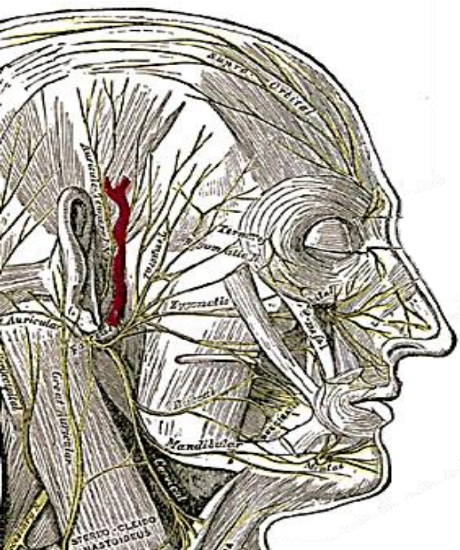
Figure 9: Extratemporal course of facial nerve
These divisions divide, rejoin and divide again to form the pes anserinus (Goose’s foot) to ultimately give the terminal branches, namely, temporal (frontal), zygomatic (malar and infraorbital), buccal, mandibular and cervical nerves (Figures 7-9).
a. Temporal (frontal) branch: This is the terminal branch of the superior division and travels along Pitanguy’s line which extends from 0.5 cm below the tragus to 1.5 cm above and lateral to the eyebrow. The nerve becomes increasingly superficial as it travels cephalad and lies just deep to the temporoparietal (superficial temporal) facia at the temple. At the level of zygomatic arch, it arborises into two to four branches to innervate the frontalis muscle from its inferior aspect. Temporal branch injury causes ipsilateral frontalis muscle paralysis.
b. Zygomatic branch: This is arguably the most important branch of the facial nerve as it supplies orbicularis oculi, which enables eye protection. Consequently, injury to the zygomatic branch may cause lagophthalmos (inability to completely close the eye) with risks of exposure keratitis, corneal ulceration and scarring. Ultimately, this may lead to blindness.
c. Buccal branch: This divides into multiple branches traveling at the level of the parotid duct. The surgical landmark to locate these branches is 1 cm or one finger breadth below the zygomatic arch. The buccal branch innervates the buccinator and upper lip musculature. It is also important for lower eyelid function, as the medial canthal fibers of buccal branch innervate the inferior and medial orbicularis oculi. Injury to the buccal branch causes difficulty emptying food from the cheek and an impaired ability to smile. However, due to the high degree of arborisation (buccal branch always receives input from both the superior and inferior divisions of the facial nerve) damage to this branch is less likely to result in a functional deficit. The zygomatic/ buccal motor branch, that innervates the zygomaticus major can consistently be found at the midpoint (Zuker’s point) of a line drawn between the helical root and the lateral oral commissure (Dorafshar et al. 2013)
d. Marginal mandibular branch: This is a terminal branch of inferior division and runs just below the border of mandible, deep to platysma and superficial to the facial vein and artery. It supplies the muscles of the lower lip (depressor anguli oris). Injury results in ipsilateral lack of depression of the lower lip and asymmetry of open mouth smiling or crying. It is connected to other rami in only 15% of cases; consequently, clinical weakness is highly likely following injury.
e. Cervical branch: This is a terminal branch of the inferior division of the facial nerve. It runs down into the neck to supply platysma (from its deep surface)
Interconnections exist between buccal and zygomatic facial nerve branches in 70-90% of patients; hence injury to these branches may be clinically compensated for by these interconnections. This is not true for the frontal and marginal mandibular branches which are terminal branches without significant crossover. Hence injuries to the frontal and marginal mandibular branches are less likely to clinically recover.
Clinically it may be possible to predict the site of facial nerve injury. Injuries at or distal to stylomastoid foramen only have paralysis of facial musculature. More proximal injuries may also manifest loss of taste and hyperacusia.
Etiology of Facial Paralysis
Congenital causes include obstetric, developmental facial paralysis, Möbius syndrome and idiopathic mandibular division palsy. Acquired causes include traumatic temporal bone fracture or facial injury, neoplastic, infectious, neuromascular and iatrogenic (parotidectomy or acoustic neuroma excision) (Westin & Zuker 2003).
Obstetric injury, often with forceps delivery, is the commonest cause of congenital cause; most recover within a month.
Idiopathic marginal mandibular nerve palsy (asymmetrical crying facies/con genital unilateral lower lip palsy/CULLP) causes unilateral weakness of depressor anguli oris and is associated with other major congenital anomalies in 10% of children, most commonly the cardiovascular system (44%). It affects about one in 160 live births.
Möbius syndrome is a rare condition of unknown origin. Children usually present at an early age with facial and ocular symptoms which include incomplete eye closure, mask-like face, inability to follow gaze, drooling and difficulty sucking. The facial nerve is always involved, the abducens nerve is involved in 75-100% of cases, and the hypoglossal nerve is commonly implicated. Other cranial nerves e.g. oculomotor, trigeminal, glossopharyngeal and spinal accessory may be involved. The condition is often bilateral, but can be unilateral, with complete or incomplete paralysis of the affected nerves. Limb abnormalities may be present in up to 25% of cases, e.g. clubbed feet, congenital hand anomalies (including syndactyly) and pectoral anomalies in 15% (Poland’s syndrome). A myriad of other associated conditions including hypotonia, hearing problems, Pierre-Robin sequence, congenital heart disease, spinal anomalies, microglossia and peripheral neuropathies are described.
Goldenhar’s syndrome comprises hemifacial microsomia, epibulbar dermoid cysts and vertebral anomalies, and may have facial nerve weakness.
Bell’s palsy accounts for 66-85% and is the commonest cause of acquired facial palsy. Etiology is unknown although polymerase chain reaction studies of viral DNA suggest that latent herpes viruses may be responsible. Swelling of the nerve within the fallopian canal may cause nerve compression and an immune reaction. This disrupts the neural microcirculation resulting in demyelination and impaired conduction of neural impulses. It has an incidence of 1 in 5000 and carries a 1 in 60 lifetime risk. It affects males and females equally, although it is associated with pregnancy and is four times more common in diabetics. It is typified by sudden onset of unilateral facial paresis or paralysis. There may be a prodromal taste disturbance due to conduction anomalies in the chorda tympani, pain, hyperacusis (due to stapedial denervation) and excessive lacrimation. Signs include brow and forehead ptosis, upper eyelid retraction, ectropion (orbicularis oculi dysfunction), decreased blink reflex and decreased ability to close the eye. Initial management may include corticosteroids and antivirals. The use of acyclovir however is controversial and systematic reviews of randomized controlled trials showed no benefit over corticosteroid therapy alone (Lockhart et al. 2009). A typical regime in adults is 30 mg prednisolone twice a day for 5 days with a tapering dose over 5 days. Particular attention should be made to eye protection using frequently applied lubricating eye drops by day and eye gel at night. All patients with Bell’s palsy begin to recover function within 6 months and almost none will remain totally paretic. However, a significant proportion has residual weakness, synkinesis (coarse, uncoordinated facial movement e.g. involuntary eye closure when smiling due to aberrant regeneration) or hemifacial spasm. Recovery begins within three weeks in 85% of patients, but only at three to six months in 15%. Most show some signs of recovery within four weeks. Earlier recovery por tends a better prognosis. Recovery continues over a period of up to 12-18 months. Predictors of a poor outcome include initial complete paralysis, no recovery at three weeks, >60 years of age and low electromyogram amplitudes. About 10% of Bell’s palsies may recur.
Trauma is the 2nd commonest cause and may be due to temporal bone fracture, penetrating wounds or obstetric injury.
Temporal bone fractures (longitudinal, transverse or mixed) are classified according to the fracture line in relation to the long axis of the temporal bone. Facial paralysis is more likely with transverse fractures. End-to-end coaptation of a disrupted intratemporal facial nerve requires an otologic approach.
With penetrating facial wounds, nerve injury to facial nerve branches medial to a line drawn vertically through the lateral canthus of the eye may retain facial movement because of the degree of arborization; nevertheless, nerves should be repaired if possible. However, injury to the frontal and marginal mandibular nerves manifesting with weakness should always be repaired as the likelihood of spontaneous recovery is poor. Nerve repair should ideally be done within 72 hours to permit identification of the distal ends of the severed nerves by electrical stimulation; beyond 72 hours neurotransmitter stores may become depleted.
Tumors may cause facial palsies and can have a variable presentation of onset (sudden or insidious), severity (complete or incomplete), number of episodes (single or multiple) and presence of facial muscle hyperkinesis. A neoplastic cause should be considered with unilateral facial weakness which slowly progresses over several weeks/months, or with an abrupt onset without return of function at 6 months, or with associated hyperkinesis. Malignancy causing facial paralysis most commonly originates in the parotid gland or may be due metastases from skin cancers. Other diagnoses include acoustic neuroma (including neurofibromatosis Type 2), cholesteatoma of the ear, and intracranial tumors.
Viral infections account for about 12% cases of facial paralysis. Ramsay-Hunt syndrome is a Varicella zoster infection causing facial paralysis, otalgia and a rash within the external auditory meatus. Treatment involves prednisolone 1 mg/kg/day divided into two doses per day. Acyclovir is recommended by some (800 mg 5 times per day for 10 days) (Gnann 2007).
Lyme disease is a tick-borne bacterial infection (Borrelia) may present with bilateral facial palsy.
HIV may cause either unilateral or bilateral facial paralysis and may be caused by toxoplasmosis, encephalitis, lymphoma, Herpes simplex and Herpes zoster infection (Ramsay-Hunt Syndrome).
Melkersson-Rosenthal Syndrome, a rare condition of unknown etiology, characterized by a triad of non-inflammatory facial edema, congenital tongue fissures (lingua plicata) and facial palsy (RiveraSerrano et al. 2014). Not all the features of the triad may be present or fully expressed. Facial palsy may be recurrent. A hereditary factor (autosomal dominant with variable expressivity) has been implicated. Treatment is usually conservative.
Assessment of Facial Palsy
Patients should ideally be assessed and managed by a multidisciplinary team i.e. otolaryngology, plastic surgery, neurosurgery, ophthalmology, psychology, speech and language therapy, occupational therapy and physiotherapy.
History
This includes demographic details, occupation, and hobbies, general past medical history, current medications, allergies, smoking and alcohol consumption.
It is important to determine when the facial palsy was first noticed; whether the onset was insidious or rapid; any deterioration or improvement; whether it is bilateral or unilateral; and whether it is complete or partial. The etiology may be relatively clear e.g. obstetric forceps or sharp trauma. Previous treatment for the palsy should be elicited.
Ask the patient (or parent) to describe what the main problems are; these may be functional, aesthetic and/or psychosocial.
Functional problems are assessed using a “top down” approach:
- Brow: Brow ptosis may cause visual occlusion, and/or an older or tired appearance which can in turn result in psychosocial or occupational problems
- Eyes: Excessive watering, dryness, irritation or impaired field of vision
- Nose: Difficulty breathing, nasolabial scoliosis
- Mouth: Labial articulation difficulty, inadvertent lip or cheek biting, drooling or problems with retropulsion of a buccal food bolus
Aesthetic problems may be a consequence of facial asymmetry at rest, possibly with severe facial droop, and an uneven or absent smile.
Psychosocial problems may include social avoidance and withdrawal, embarrassment of eating in public, avoiding being photographed, relationship problems, anxiety or depression.
Examination
Inspect the face to assess skin quality, gross symmetry between the two sides of the face, evidence of scars or previous trauma and the presence of synkinesis at rest. Assess the integrity of each facial nerve division by asking the patient to raise their eyebrows, close their eyes, blow out their cheeks, show their teeth, and smile.
Use a “top down” approach; divide the face into upper, middle and lower thirds:
Upper third:
- Forehead: Presence or absence of wrinkles and position of the brow
- Upper eyelid: Position and dermatochalasis. Assess movement and strength of eye closure. Measure lagophthalmos (inability to completely close the eye) with a handheld ruler
- Lower eyelid: Inspected for ectropion. Determine the laxity of the lower lid by doing a “snap-test”; gently pull the lower eyelid downward and away from the globe for several seconds, releasing the lid and seeing how long it takes to return to its original position without the patient blinking. Normally, the lower eyelid springs back almost immediately to its resting position. The more time it takes for the lid to resume its normal position the greater the lid laxity
- Conjunctiva: Check for exposure i.e. inflammation and dilated vessels
- Bell’s phenomenon: Check for the presence or absence of a Bell’s phenomenon. It refers to upward and outward movement of the globe when an attempt is made to close the eye, is present in 75% of people, and is a defensive mechanism to protect the cornea. Absence thereof puts the cornea at risk of desiccation.
Middle third:
- Nasal airways: Assess each nose to exclude rhinitis or fixed nasal obstruction. Use Cottle’s manoeuvre (lateral traction at alar base improves heminasal breathing) to collapse of the internal nasal valve
- Cheek: Check for ptosis and asymmetry of the nasolabial crease
- Mouth: Determine the resting position of the mouth and for commissural droop. Check for contralateral philtral deviation; in congenital conditions this is often pulled to the normal side, whilst in resolved/resolving acquired conditions it is often to the affected side due to hypertonicity
- Oral commissure excursion: Measure commissural excursion with a handheld ruler
- Lower lip: Observe for weakness of the depressor anguli oris due to marginal mandibular nerve weakness
- Upper teeth: Degree of upper tooth show
- Smile: Document the type of smile (Rubin 1974; Paletz et al. 1994; Manktelow et al. 2008)
Lower third:
- Chin: Note the position of the chin.
- Platysma: This is assessed by asking the patient to pretend to shave. At this point it is important to look for the presence of synkinesis.
Functional assessment (speech, drinking and eating) is performed. Medical photography or videography can provide a preoperative baseline.
Electromyography may provide prognostic information in individuals with acute complete (<18 months) or partial facial palsies but is rarely meaningful in those with an established paralysis.

Table 1: The Sunnybrook Facial Grading System provides a composite score (based on voluntary movement, resting symmetry and synkinesis) which may be used to monitor recovery after facial nerve injury
Grading scales, such as the Sunnybrook Facial Grading system (Table 1) which assesses resting symmetry, voluntary movement and degree of synkinesis, may be of benefit. They provide longitudinal, numerical data, which may help quantitate post-intervention changes.
The House-Brackmann scale is unhelpful to report postoperative outcomes as it is subjective and was not designed to report reconstructive outcomes (House & Brackmann 1985). The FaCE (Facial Clinimetric Evaluation) scale and videographic analysis may also be used (Kahn et al. 2001; Frey et al. 1999). The FaCE Scale comprises 51 items: 7 visual analogue scale items to measure patients' perceptions of broader, global aspects of facial dysfunction and 44 Likert scale items rated from 1 (worst) to 5 (best) to measure patients' perceptions of specific aspects of facial impairment and disability.
Finally, examine the patient with a view to corrective surgery. Examine the nerve supplies (trigeminal nerve; CN V) to the temporalis and masseter muscles; and the spinal accessory (CN XI) and hypoglossal (CN XII) nerves to determine their utility as donor nerves. Palpate the superficial temporal and facial vessels or use a handheld Doppler to assess their suitability as recipient vessels if free tissue transfer is to be done.
Management of Facial Palsy
Management can be non-surgical or surgical. It should be tailored to the individual to restore function, regain resting symmetry and dynamic, spontaneous movement. Determining which strategy to pursue requires a multidisciplinary approach and intimate involvement of the patient and the family. The patient’s age, prognosis, and functional, aesthetic and psychological concerns are important considerations. These may take some time to be fully elicited so patience, empathy and sensitivity are paramount. Repeated consultations are often necessary.
Non-Surgical Treatment
The first priority is to protect the eye. This may be achieved by regular use of lubricating drops and gel, protective glasses, and taping the eyelids. A humidification chamber may benefit less compliant patients. Horizontal taping of lower lids may aid paralytic ectropion.
Botulinum toxin can be employed on the normal side to improved symmetry; it paralyzes selected muscles by disrupting acetylcholine release from motor nerve endplates. E.g. if the marginal mandibular branch is damaged then depressor anguli oris denervation can result in an asymmetrical lower lip, which “rides high” on the affected side and is particularly noticeable on smiling. If this occurs in a neonate it is known as crying facies syndrome. Reducing the activity of the normal depressor anguli oris may improve symmetry. Botulinum toxin is also used to weaken the contralateral frontalis muscle (Moody et al. 2001); and can also be used to treat spasticity, such as platysmal bands, and synkinesis (Maio and Bento. 2007).
Other non-operative interventions include physiotherapy, mime therapy and speech therapy.
Psychological support and social skills training for people with facial disfigurement has many advocates (MacGregor 1990; Kapp-Simon et al. 1992). This includes the use of cognitive behavioural therapy, anxiety-management techniques and communication workshops (Partridge 1994; Kent 2003).
Surgical Treatment
The aims of operative treatment are to protect the eye, restore facial symmetry, facilitate a spontaneous, dynamic smile and improve speech. Operative procedures may be static or dynamic.
Static Procedures
Static procedures do not reproduce the dynamic movement of the face, although it may be ameliorated. Static procedures may be very beneficial to provide corneal protection, improve the nasal airway, prevent drooling and improve facial asymmetry at rest. They are potentially indicated in the elderly, the unfit, those unwilling or unfit to undergo prolonged surgery, those with established paralysis without viable facial musculature, as a temporizing measure, with massive facial defects secondary to trauma or cancer extirpation, or after failed microvascular procedures.
Static procedures to correct functional disabilities include temporary or permanent tarsorrhaphy; insertion of gold weights, palpebral springs or lid magnets; Müllerectomy; brow lift or suspension; forehead skin excision; unilateral facelift type procedures; and static slings.
Temporary tarsorrhaphy: Temporary tarsorrhaphy (lateral or medial) narrows the palpebral fissure by approximating parts of the eyelids. It aids eyelid closure and may be performed if there is an expectation of recovery. It may be indicated if ocular surface symptoms do not improve or if definitive surgical correction is not achieved within a few weeks.
Permanent tarsorrhaphy: This is a definitive solution, but because of the significant disfigurement it causes, it is rarely performed. It may be indicated if there is little expectation of recovery of eyelid closure. An example is the McLaughlin tarsorrhaphy, a lash preserving procedure, where flaps are elevated from the lateral thirds of the eyelids. The flaps are posteriorly based on the lower lid and anteriorly based on the upper eyelid and are sutured together.
Gold weights (1-1.6g), palpebral springs or lid magnets: Insertion of a gold weight into the upper eyelid may aid eye closure. Platinum chains, palpebral springs and lid magnets have also been described. These procedures are generally technically easy to do and can be reversed or modified. Good symptomatic relief from corneal exposure and decreased burden of care are often achieved by using gold weights. Gold weights are placed immediately in front of, and sutured to, the tarsal plate. Consequently, they are visible through the skin. Gold is generally used as it is skin colored, comfortable and inert. However, there is a high extrusion rate (approximately 10%) after 5 years (Rofagha and Seiff 2010). With longer follow-up the exposure rate continues to increase. However, extruded weights may be replaced many times and often continue to provide good benefit.
- Select the appropriate gold weight preoperatively by taping a series of implants in 0.2 gram increments to the outside of the upper lid until the patient is able to achieve complete lid closure. Permit the patient to wear the weight for 15 minutes and check for comfort and size. Check that lid closure is achieved when lying supine
- Surgery is done under local anesthesia
- Mark the supratarsal crease about 10 mm from the inferior lid margin
- Inject local anesthetic/vasoconstrictor into the upper lid
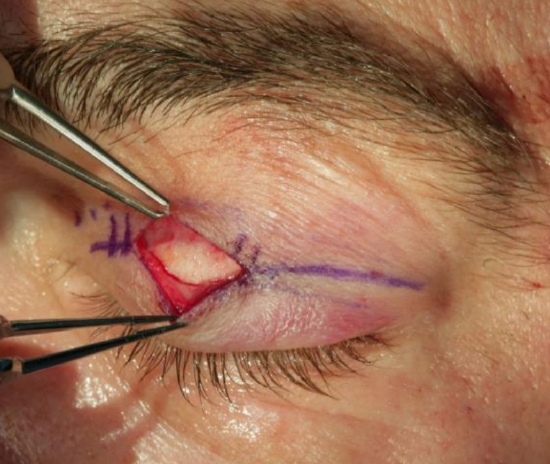
Figure 10: Tarsal plate exposed
- Make a 2 cm incision in the supratarsal crease (Figure 10)
- Cut through the orbicularis oculi muscle and expose the tarsal plate (Figure 10)
- Create a pocket for the implant between the orbicularis oculi and the tarsal plate
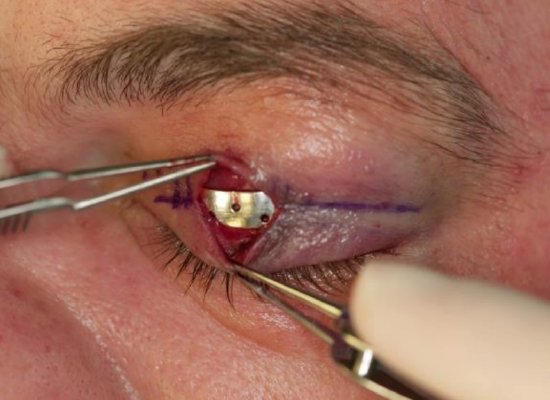
Figure 11: Gold weight centered over the medial limbus of the iris
- Center the gold weight over the medial limbus of the iris (Figure 11)
- Fix the weight by passing clear 6-0 nylon sutures through the holes in the weight and with partial thickness sutures in the tarsal plate
- Inspect the underside of the eyelid to ensure that the conjunctiva has not been breached
- Repair the orbicularis oculi muscle with interrupted 5-0 vicryl sutures
- Close the skin with 6-0 fast absorbing sutures
Müllerectomy: This may be a more suitable alternative to gold weight insertion in some individuals. It is performed by advancing the levator aponeurosis and may drop the upper eyelid by 2-3 mm. A successful procedure requires the combined function of the levator and Müller’s muscles (Scuderi et al. 2008); hence it requires good muscle function. Müller’s muscle is an involuntary and sympathetically innervated muscle. It originates inferior to the levator aponeurosis distal to Whitnall’s ligament, and attaches to the cephalad tarsal border, and is estimated to produce 2-3 mm of eyelid elevation (Mercandetti et al. 2001). The conjunctiva and muscle are separated from the levator aponeurosis. The anterior aponeurosis is bluntly dissected and separated from the orbicularis muscle. The aponeurosis and Müller’s muscle are then sutured to the anterior portion of the tarsal plate (Mercandetti et al. 2001; Scuderi et al. 2008). Complications (Mercandetti et al. 2001) include: excessive or inadequate correction; corneal abrasion; ulceration; hemorrhage and infection Because a Müllerectomy does not allow for intraoperative adjustment of upper eyelid height, unlike a levator resection, the estimation of the resection length is made preoperatively using various reported ratios of muscle resection to eyelid elevation (1 mm resection for 0.32 mm elevation was reported by Mercandetti et al. 2001).
Brow lift / Suspension: These are used to elevate a ptotic brow caused by a paralyzed frontalis muscle. Brow lifts not only improve the aesthetic appearance but remove obstruction to the upper visual field. Endoscopic or open techniques can be used, and scars are often inconspicuous. The lift achieved is less than that achieved with a direct lift achieved with the forehead skin excision technique. Transpalpebral browpexy has also been described which has the advantage of placing the scar within the upper eyelid (Niechajev 2004).
Forehead skin excision: A melon-slice shape of skin may be excised immediately above the eyebrow or in the temple. It is probably most effective in patients with complete facial palsy and brow ptosis. It produces a visible scar and is usually performed in older patients.
Unilateral facelift-type procedure: Cosmetic facelifts have been described for facial palsy. They remove excess skin and improve facial symmetry; however, results probably only last for the short to medium term. Other procedures include a subperiosteal facelift with lower lid rebalancing and a suborbicularis oculi fat lift (Ramirez et al. 1991; Horlock et al. 2002). These are probably more effective techniques and raise the suborbicularis fat in conjunction with the origins of the zygomaticus and levator muscles of the lip and suspend them from the deep temporal fascia. Thread-lift and endotine lifts have also been described although the results are still in their infancy (Stephens et al. 2009).
Static slings: Airway obstruction due to hypotonia of the nasal musculature is often overlooked but may have a significant functional effect and should be evaluated and addressed. It may be aggravated by rhinitis or a fixed nasal airway obstruction.
Tensor fascia lata (TFL) and palmaris longus: Gore-Tex/polytetrafluoroethylene slings have also been described but are associated with high complication rates. These may be used to support several structures:
- Lower eyelid: Ellis and Kleiman (1993) described a Gore-Tex sling, tunneled subcutaneously from the anterior lacrimal crest to the zygomatic process to suspend the medial lower lid. Tension on the sling elevates the lid and positions the punctum against the globe.
- Recreate the nasolabial fold
- Elevate corner of the mouth (Leckenby et al. 2013)
- A 5 cm x 25 cm of TFL is usually harvested
- Incise the face using the routine facial palsy incision with a parotid extension
- Raise a skin flap up to the oral commissure to facilitate exposure of the modiolus and to avoid an additional nasolabial incision
- Make 2 vertical stab incisions in the midline of the upper and lower lips
- Tunnel the upper slip of the TFL graft to the midline of the upper lip
- Pass this slip around the orbicularis oris and suture it in place with a non-absorbable suture (e.g. 4-0 prolene)
- Secure the lower slip to the lower lip; this should be inset roughly 1 cm tighter than that of the upper lip and acts to effectively support the lower lip
- Anchor the final slip to the orbicularis oris at the modiolus
- Attach the proximal end of the graft under tension to the temporalis and parotid fascia
- Elevate the cheek and nose and draw the nasal ala laterally to open the external nasal valve
- The static slings are passed subcutaneously from the corner of the mouth, ala of the nose or cheek and anchored onto the deep temporalis fascia
- A superior vector acting on the oral commissure can improve oral competence and bilabial speech and achieve resting symmetry
Dynamic Procedures
Dynamic procedures attempt to improve facial symmetry in repose and to provide synchronous facial movement, preferably in a spontaneous manner.
The authors’ reanimation strategy for facial paralysis is generally as follows:
- “Early” (usually < 1 yr after injury): nerve-based reconstruction
- "Late" (usually > 1 yr after injury) or developmental: muscle-based reconstruction; preferably segmental free muscle transplants for versatility and precision
- “Longstanding”: segmental free muscle transfers for versatility, precision and spontaneity
- If the contralateral facial nerve is healthy then this is used to power a cross-facial nerve graft. If there is no healthy contralateral nerve or the patient has bilateral facial palsy, then the nerve to masseter can be used
Nerve repair
Direct nerve repair should be performed before the nerve ends retract. Depending on the nature and location of injury, facial nerve repair should be attempted at the time of, or as soon as possible after the event. Exploration within 48-72 hours permits identification of the distal nerve stump by using a nerve stimulator, thereby reducing the probability of erroneous nerve repair. Although nerve repair should be undertaken within one-year, good results have been reported up to three years following injury. Nerves regenerate at approximately 1 mm per day after an initial period of two to four weeks of no growth. Close observation is probably the best management for blunt facial nerve injury although synkinesis may result from axonotmesis.
- A ‘less is more’ approach is probably best: accurate, tension free coaptation with minimal epineural sutures are the hallmarks of sound direct nerve repair
- A key principle is to have no tension (breakage of 8-0 gauge suture is a traditional rule of thumb that the repair is under too much tension; if there is undue tension, then another means of repair, e.g. nerve grafting, is used
- Minimally debride the nerve ends to expose the epineurium
- Free the nerve from some of the surrounding tissues both proximally and distally from the site of injury; but this should not be extensive (< 2 cm) as this might devascularize the nerve
- Orientate the fascicles and match them as accurately as possible
- Epineural nerve repair is performed using an 8-0 or finer gauge suture
- If possible, this is performed under an operating microscope, or with high powered loupes
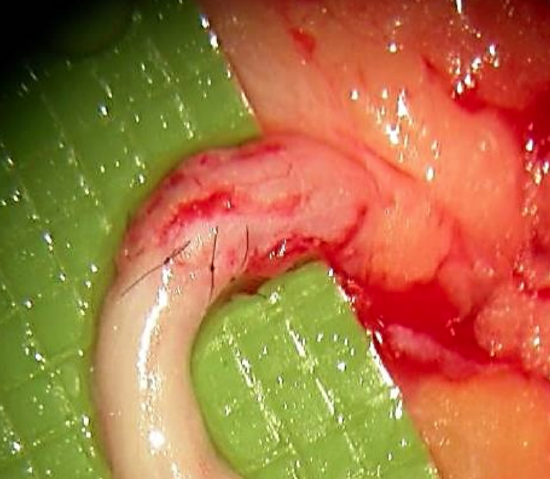
Figure 12: Close-up view of completed nerve repair
- Use the least number of sutures to achieve accurate coaptation (Figure 12)
Harvesting nerve grafts
The greater auricular nerve, sural nerve and branches of the cervical plexus are potential donor nerves.
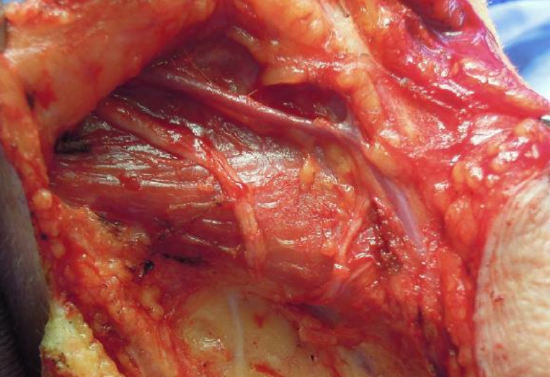
Figure 13: Greater auricular nerve runs parallel to external jugular vein. Note transverse cervical nerves coursing anteriorly from Erb’s point
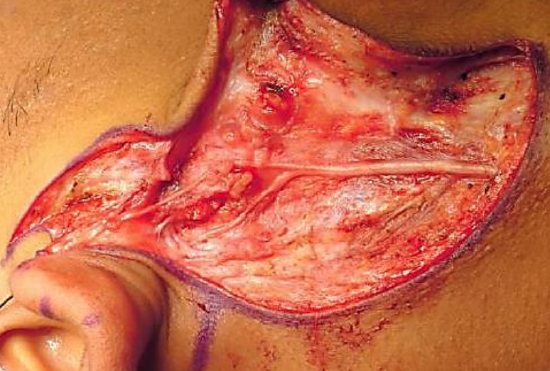
Figure 14: Greater auricular nerve dividing into two branches
Greater auricular nerve: This nerve is well-matched to the facial nerve diameter, is in the same surgical field, and leaves patients only with sensory loss of the inferior 2/3 of the auricle and over the angle of the mandible. It is found just deep to platysma, and runs superiorly over the sternomastoid muscle from Erb’s point (one-third of the distance from either the mastoid process or the external auditory canal to the clavicular origin of the sternomastoid muscle) parallel and 1-2 cm posterior to the external jugular vein (Figure 13). It generally divides superiorly into two branches that can be anastomosed to two branches of the facial nerve (Figure 14).
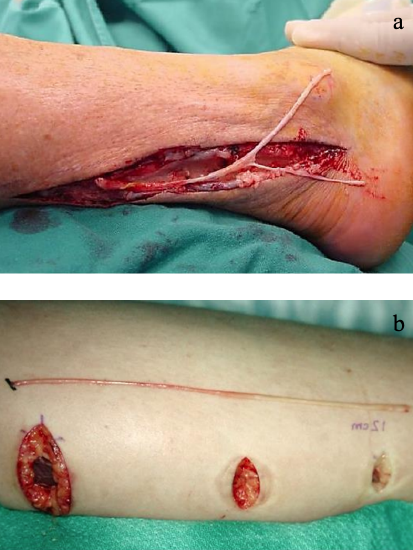
Figures 15a, b: Sural nerve harvesting techniques
Sural nerve: Being distant to the face it facilitates a two-team approach, is well-matched to the facial nerve diameter, and leaves minimal donor site morbidity (scars are often inconspicuous and the patients are usually left with sensory loss on the lateral border of the foot) (Ijpma et al. 2006). It is located posterior to the lateral malleolus and has several branches and is of greater length that the greater auricular nerve making it better suited to bridging longer defects and for grafting to more peripheral branches (Figure 15). The nerve is harvested as follows:
- A longitudinal incision or transverse "step ladder" incisions may be used (Figures 15a, b)
- Make the initial incision behind the lateral malleolus
- Identify the nerve approximately 2 cm behind and 1-2 cm proximal to the lateral malleolus
- If the small saphenous vein is found, the nerve will be located medial to it
- If using stepladder incisions, make another small incision at the junction of the middle and distal third of the leg and identify where the lateral sural cutaneous nerve branches off the medial sural cutaneous nerve
- Divide the lateral sural cutaneous nerve from the medial sural cutaneous nerve
- Dissect the medial sural cutaneous nerve proximally to just below the popliteal fossa
- This provides 30-35 cm of nerve for grafting
- Transect the nerve proximally and wrap it in a moist gauze swab
Primary nerve grafting
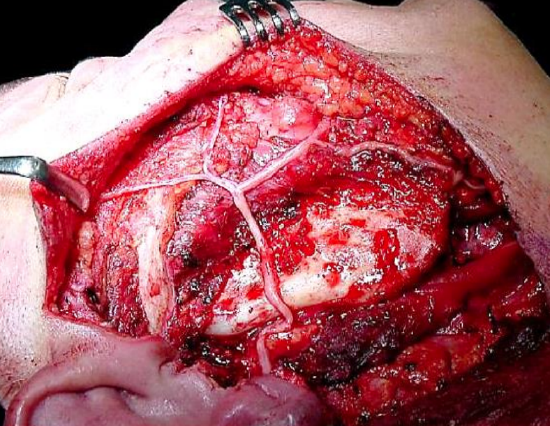
Figure 16: Sural nerve graft following facial nerve resection for a malignant parotid tumor
Nerve grafting should be performed if there is tension on a direct repair as tension reduces neuronal sprouting (Sunderland et al. 2004). Breakage of an 8-0 gauge suture when attempting primary neurorrhaphy suggests that the repair is under excessive tension. As a general rule nerve grafting should be used to provide coaptation if the gap between the two facial nerve stumps is greater than 2 cm. This may occur, for example, following nerve resection during tumor removal (Figure 16). Grafting may be attempted three weeks to one year after injury. After this time meaningful reinnervation is unlikely to be successful due to motor endplate degeneration and facial muscle atrophy. There is likely to be a 6- 24-month interval before any functional recovery is observed.
Cross-facial nerve grafting (CFNG)
The CFNG harnesses neuronal activity from the uninjured facial nerve activity to the contralateral side to power a free muscle transfer. It is probably the gold standard to accomplish symmetrical, spontaneous facial movement. However, a CFNG alone is usually not powerful enough to produce an adequate smile.
Indications for CFNG include:
- A distal stump is present
- Complete transection when the ipsilateral proximal facial nerve stump is unavailable for grafting
- The facial muscles are capable of useful function following reinnervation (probably <1 year post-injury before motor endplate degeneration is likely to have occurred)
Numerous techniques have been described (Scaramella 1971; Fisch 1974; Scaramella 1975); Anderl 1979; Baker & Conley 1979a). A variety of techniques have been described relating to surgical exposure of the donor and recipient nerves, the length and positioning of jump grafts, and timing of 2nd stage neurorrhaphy in the VII-VII CFNG technique. Because of inadequate data, it is difficult to determine the best technique (Fisch 1974; Smith 1971; Anderl 1977).
CFNG is probably most useful in association with other reanimation techniques to address a single territory within the face rather than to reinnervate the entire contralateral facial nerve e.g. using it for an isolated marginal mandibular nerve paralysis (Terzis and Kalantarian 2000).
CFNG may also be useful with a partial facial palsy to enhance residual function. A sural nerve graft may be used to connect healthy peripheral nerve branches on the normal side to corresponding branches supplying specific muscle groups on the paralyzed side. This can be performed using an end-to-side coaptation on the weakened or paralyzed side to minimize reduction of the remaining function on the affected side (Frey et al. 2006; Fattah et al. 2011). Less success has been reported with reinnervation of the marginal mandibular or temporal branches, although it may provide adequate tone (Fattah et al. 2012). Limited success has been reported with this technique as facial musculature usually reinnervates poorly with time and the results are inconsistent.
CFNG may be done as a one- or two stage procedure
One-stage CFNG: Both ends are repaired at the same operation.
Two-stage CFNG
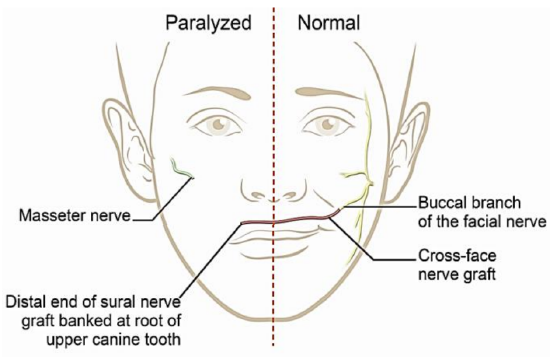
Figure 17: 1st stage of 2-stage CFNG
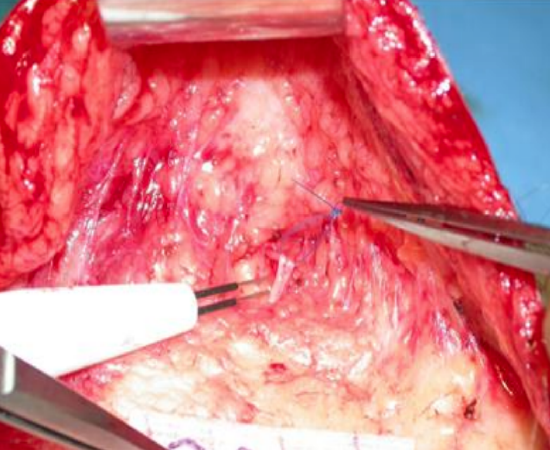
Figure 18: Identifying a buccal branch using electrical stimulation
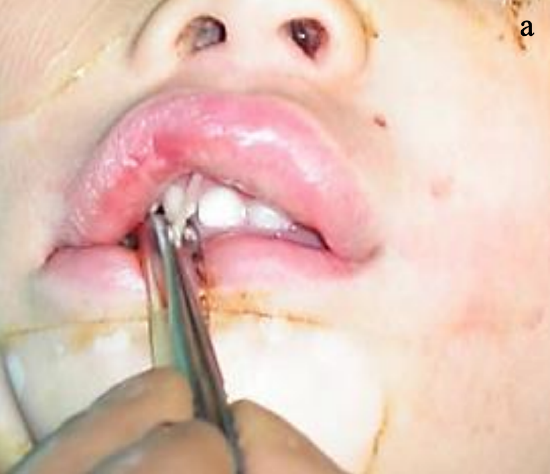
Figure 19a: End of sural nerve
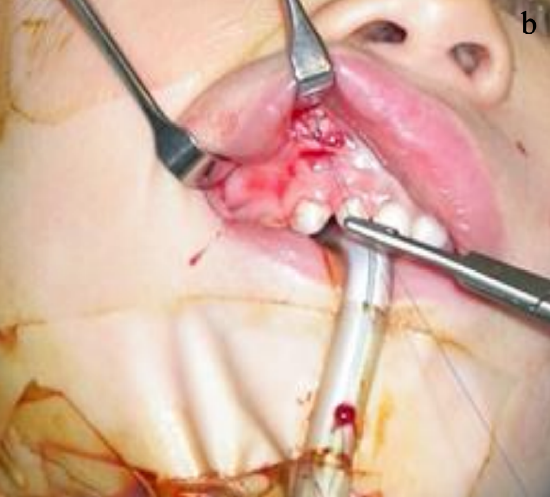
Figure 19b: End of sural nerve sutured into a pocket created above the upper canine
Figure 20: Coaptation of sural nerve graft to the functioning facial nerve
- 1st stage (Figure 17)
- Harvest a sural nerve graft (Figures 15a, b)
- Identify a suitable buccal branch of the functioning facial nerve via a nasolabial fold or preauricular incision (Figure 18)
- Tunnel subcutaneously from the buccal branch across the face to the root of the upper canine tooth on the non-functioning side
- Pass the sural nerve along the tunnel
- Suture the end of the sural nerve to the tissues in the pocket in the buccal sulcus above the upper canine to avoid it retracting (Figures 19a, b)
- Coapt the other end of the sural nerve to the end(s) of the freshly divided edges of one or several buccal branches of the functioning facial nerve (Figure 20)
- 2nd stage
- This is often performed 9-12 months following the 1st stage
- A positive Tinel’s sign can be elicited at the end of the nerve graft (tapping on the graft produces a tingling sensation); this indicates the presence axonal regeneration and that the nerve fascicles have reached the end of the nerve graft
- The surgery is done only on the paralyzed side
- Resect the terminal neuroma on the sural nerve
- Suture the graft to distal (paralyzed) stump of facial nerve
Nerve transfers
Nerve transfers are relatively easy to do and require nerve regeneration only over a single neurorrhaphy. They therefore usually provide powerful reinnervation, and good muscle tone and powerful excursion. However, nerve transfer may produce grimacing, mass facial movement and synkinesis; this may be palliated with botulinum toxin. The function of the donor nerve is also sacrificed.
Potential indications for nerve transfers include:
- The distal stump is present
- Proximal, ipsilateral facial nerve stump is unavailable for grafting
- Facial muscles are capable of useful function after reinnervation
The distal facial nerve stump may be coapted to:
- Hypoglossal nerve (most common)
- Nerve to masseter
- Glossopharyngeal nerve
- Accessory nerve
- Phrenic nerve
Hypoglossal nerve transfer
The hypoglossal nerve transfer is best suited to providing input to the facial nerve following extirpation of tumors involving the facial nerve proximally. When successful, intentionally manipulating the tongue causes facial movement. It can provide excellent tone, a normal appearance at rest in 90% of patients, and protection of the eye. It also permits intentional facial movement. However, unlike CFNG, changes in facial expression are not spontaneous. Recovery generally occurs over 6-24 months and may be observed for up to 5 years. Outcomes are variable.
The time interval between initial denervation and nerve transfer is the primary marker of success. It would appear that reinnervation must occur within 2 years after injury, otherwise atrophy and neuromuscular fibrosis mitigates the reestablishment of meaningful tone and movement (May 2000). Paralysis and atrophy of the ipsilateral tongue occurs due to its denervation. This may be marked in up to 25% of patients leading to speech and swallowing impairment (Conley & Baker 1979). Hypoglossal nerve transfer is contraindicated for the same reason in patients at risk of developing other cranial neuropathies e.g. with neurofibromatosis type II, or patients with concomitant, ipsilateral low cranial nerve dysfunction (CN IX, X, XI) palsies. A combined CN X-XII deficit may cause profound swallowing dysfunction.
To combat the above functional problems, the following hypoglossal nerve transfer techniques have been described and are described below.
Hypoglossal nerve transfer: Common initial steps
- Expose the trunk of the facial nerve as described in the Parotidectomy chapter
- Identify the pes anserinus
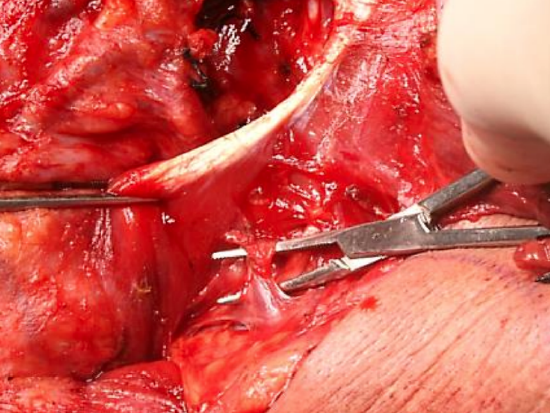
Figure 21: Divide the veins that cross the XIIn
Figure 22: Sternomastoid branch of occipital artery tethering the XIIn
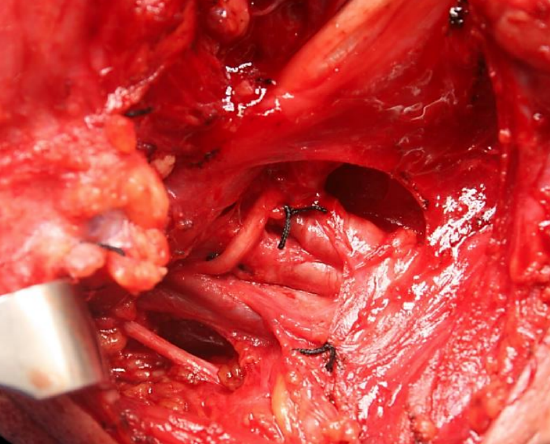
Figure 23: Dividing the sternomastoid branch of the occipital artery frees the XIIn and leads the surgeon directly to IJV. Note the XIn behind IJV
- Identify the horizontal portion of the hypoglossal nerve just inferior to the posterior belly of the digastric and the greater cornu of the hyoid bone (Figures 21-23)
- Expose the hypoglossal nerve posteriorly by dividing the lingual veins (Figure 21)
- Identify and divide the sternomastoid branch of the occipital artery; this frees up the vertical portion of the hypoglossal nerve and leads one up to the anterior aspect of the internal jugular vein (Figures 22, 23)
Classic hypoglossal nerve transfer technique (Figure 24)
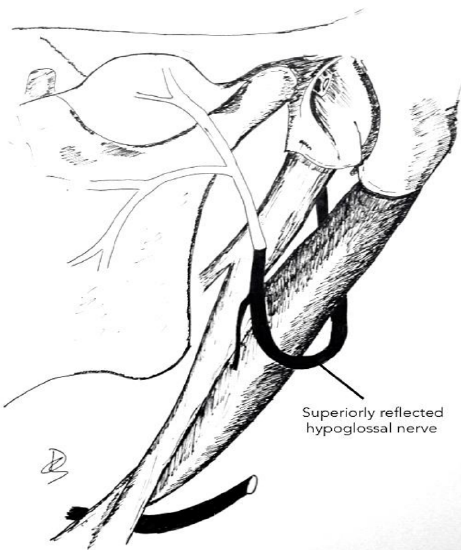
Figure 24: Classic hypoglossal nerve transfer technique (from Hadlock et al. 2004)
- Divide the facial nerve close to the stylomastoid foramen
- The facial nerve can be further mobilized by dissecting it freeing it from the parotid distal to its bifurcation
- Reflect the distal trunk of the facial nerve inferiorly
- Sharply divide the hypoglossal nerve quite far anteriorly along its course to secure an adequate length of nerve to transfer
- Reflect it superiorly up to the distal facial nerve stump
- Coapt the hypoglossal and facial nerves with 5 to 7, 10-0 nylon epineural microsutures
Split hypoglossal transfer technique (Figure 25)

Figure 25: Split hypoglossal transfer (from Hadlock et al. 2004)
The split hypoglossal nerve transfer is designed to reduce mass movement (synkinesis). Approximately 30% of the diameter of the hypoglossal nerve is divided from its parent trunk for several centimeters. This is reflected superiorly. As it provides fewer axons, it is best connected only to the lower division of the facial nerve (Conley & Baker, 1979). Other techniques may then be used to address the upper face.
Hypoglossal jump graft techniques (Figure 26)
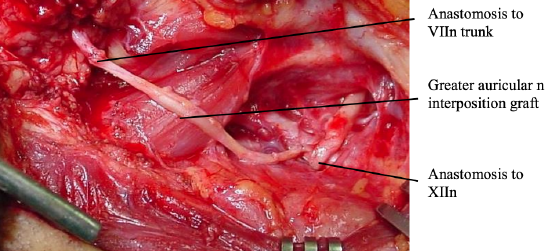
Figure 26: Hypoglossal jump graft
This is identical to hypoglossal nerve transfer, except that it involves partial sectioning of the hypoglossal nerve, and performing an end-to-side neurorrhaphy between the hypoglossal nerve and a donor nerve graft which is then connected to the distal facial nerve, thereby preserving ipsilateral hypoglossal function (May et al. 1991). It can be used when there is ipsilateral lower cranial nerve dysfunction or if the patient is unwilling to accept tongue dysfunction.
- Excise a patch of epineurium from the hypoglossal nerve
- Make a 30% incision into the hypoglossal nerve and allow the defect to splay open
- Lay the recipient nerve (sural or greater auricular) into the defect, facing the proximal cut surface, and secure it with microsutures
- Alternatively, the distal stump of the facial nerve can be directly sutured to the hypoglossal nerve. Additional facial nerve length can be gained by mobilizing the mastoid segment of the facial nerve as far as the 2nd genu, and/or freeing it from the parotid tissue distal to its bifurcation
Other attempts to preserve the hypoglossal function include the use of end-to-side coaptation (Venail 2009)
Babysitter procedure
A cranial nerve (usually hypoglossal) is transferred to achieve quicker reinnervation and to preserve musculature and potentially the denervated stump while axons migrate across the CFNG (Terzis & Tzafetta 2009)
Nerve to masseter reanimation technique
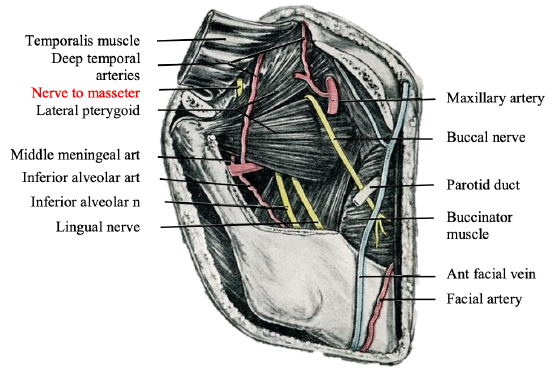
Figure 27: Zygoma, masseter and mandible removed to demonstrate the nerve to masseter
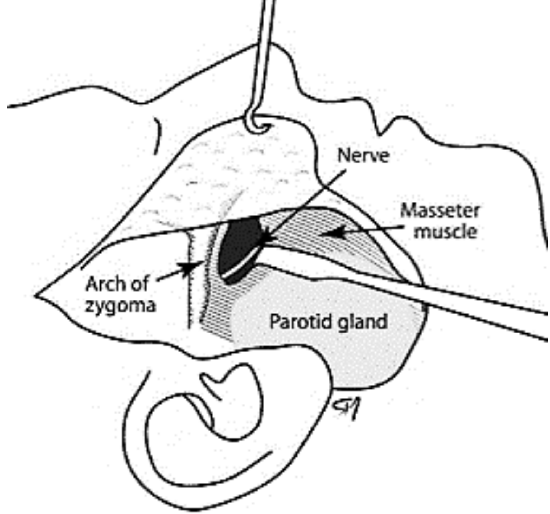
Figure 28: Nerve-to-masseter
The motor nerve to the masseter muscle is a branch of the mandibular division of the trigeminal nerve (Figure 27). It is increasingly being used for facial reanimation and is the senior authors’ preferred option due to its minimal donor morbidity (Manktelow et al. 2006). Its anatomical location is consistent (Borschel et al. 2012) (Figure 28)
- Make a preauricular incision and elevate a skin flap over the parotid (Figure 28)
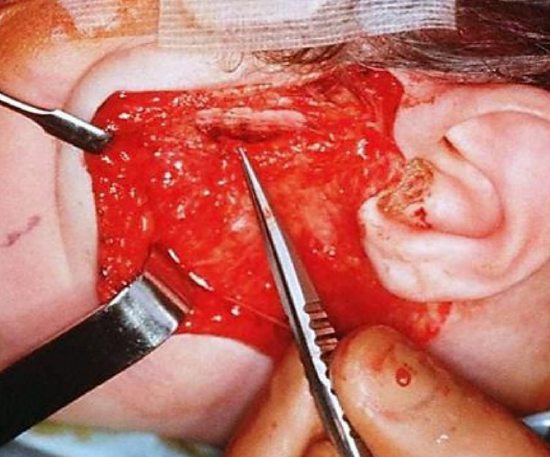
Figure 29: Nerve to masseter is located 3 cm from the tragus and 1 cm below the zygoma
- Make a transverse incision in the parotid capsule approximately 1 cm below the zygomatic arch and 3 cm anterior to the tragus (Figure 29)
- Bluntly dissect through the parotid tissue up to the surface of the masseter muscle to avoid transecting branches of the facial nerve
- Split the masseter to gain access to the deep surface of the muscle
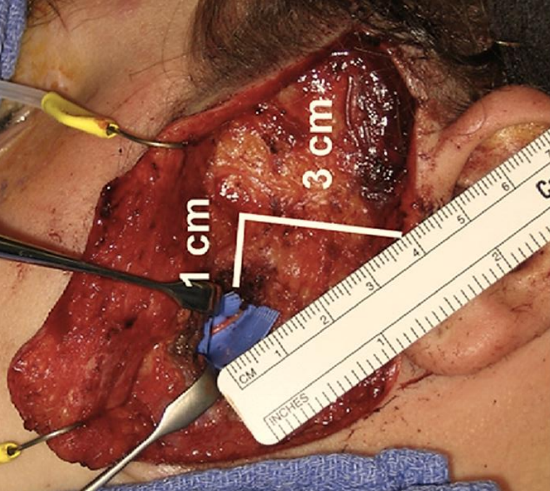
Figure 30: Nerve to masseter
- Use a nerve stimulator to locate the nerve; the nerve is generally located approximately 1.5cms deep to the superficial muscular aponeurotic system (SMAS) (Figure 30)
- Follow the nerve anteriorly until it ramifies
- There is usually a branch-free segment measuring 1 cm that can be cleanly transected distally and reflected laterally into the wound, ready for coaptation to the stump of the facial nerve
Local muscle flaps
Local muscle flaps e.g. masseter and temporalis flaps may be used when there is an absence of suitable mimetic muscles after long-standing atrophy with no potential for useful function after reinnervation. They can also be used as an adjunct to the mimetic muscles to provide new muscle and myoneurotisation.
Masseter flap (Figure 31)
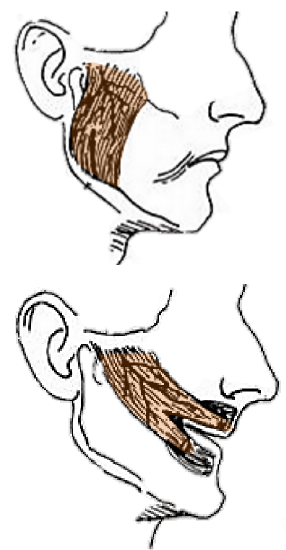
Figure 31: Masseter flap
All or part of the masseter can be used as a local muscle flap for facial reanimation.
It may be performed via intraoral approach (Adams 1947; Sachs and Conley 1982). For example, the muscle’s insertion can be detached from the lower mandibular border, transposed anteriorly, divided into three slips and inserted into the dermis above the lip, at the oral commissure and below the lip (Baker and Conley 1979b) (Figure 31). This procedure gives motion to lower half of the face and usually achieves good static control. However, the lateral vector yields an unnatural smile. A useful clinical situation would be during a radical parotidectomy, where the masseter is already exposed and can be transposed and interdigitated into freshly denervated mimetic muscles to provide maximal myoneurotisation.
Temporalis Myoplasty (Gilles 1934; Labbé and Huault 2000; Sherris 2004; Nduka et al. 2012)
Temporalis myoplasty involves detaching the insertion of the temporalis muscle to the coronoid process of the mandible and transposing and inserted it around the eye and the mouth. Sir Harold Gilles (1934) originally described the temporalis muscle flap with fascia lata grafts to reach the oral commissure.
The temporalis muscle is innervated by the trigeminal nerve. The muscle can be used to provide static support to the oral commissure and trigeminal-innervated dynamic movement. It is a good option for smile reinnervation in the chronically paralyzed face. It may also be employed as a temporising measure when the regenerative potential of the facial nerve is in doubt (e.g. following skull base surgery), or during the waiting period for regeneration, as it does not interfere with any potential facial nerve regeneration (Cheney et al. 1995). It may also be used to manage the upper face in conjunction with a hypoglossal nerve transfer to the lower division of the facial nerve.
Before embarking on temporalis myoplasty, its neurovascular supply must be confirmed to be intact as neurotologic procedures may damage these structures and congenital facial palsy syndromes may be associated with other cranial nerve anomalies that may affect temporalis function. Severe temporalis atrophy, as may found in the edentulous patient, is a further contraindication.
Temporalis myoplasty has been modified by many authors (Andersen 1961; Baker & Conley 1979b; Burggasser et al. 2002) but probably has been best popularized by Daniel Labbé (Caen, France). Labbé and Huault (2000) originally approached the temporalis muscle using a bicoronal incision, performing an anterograde dissection of the temporalis from its origin in the temporal fossa. This negated the need for any grafts as the temporalis muscle could slide forward to reach the mouth once the temporalis had been freed from its insertion on the coronoid process. The zygomatic arch was detached using two osteotomies and the tendinous part of the temporalis, now detached from the coronoid process, was tunneled through the buccal fat pad. Others have modified the procedure to allow a more limited incision directly over the temporalis and without a need for zygomatic osteotomies.
A video of lengthening temporalis myoplasty technique
Although temporalis myoplasty provides good static control, it has been criticized as it is generally unable to recreate a spontaneous, dynamic smile as its activation often requires clenching of the teeth (Fattah et al. 2012).
Effective rehabilitation through training and physical therapy is necessary to optimize outcomes. The literature supports the theory of cortical plasticity whereby following a rehabilitation period, individuals with trigeminally innervated muscle transfers perform facial movements without having to clench their teeth. There are also reports of subconscious smiling with this technique. Whether this is a central or a peripheral phenomenon is unclear (Rubin 1999). CFNGs coapted to the contralateral facial nerve may help synchronize smiling on both sides of the face (Freilinger 1975; Terzis and Kalantarian 2000).
Temporalis Lengthening Myoplasty: Surgical Technique
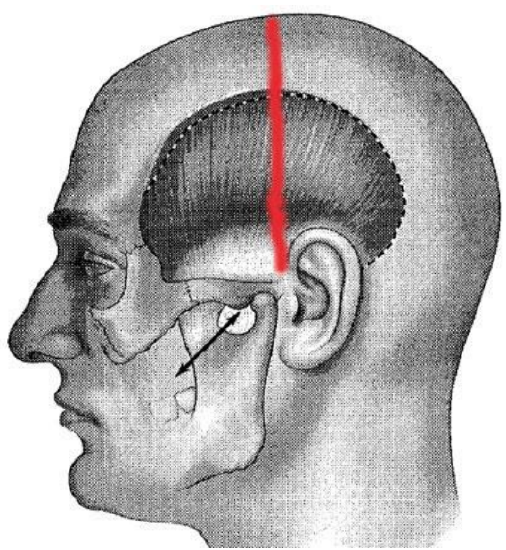
Figure 32: Original bicoronal incision (Labbé 2000)
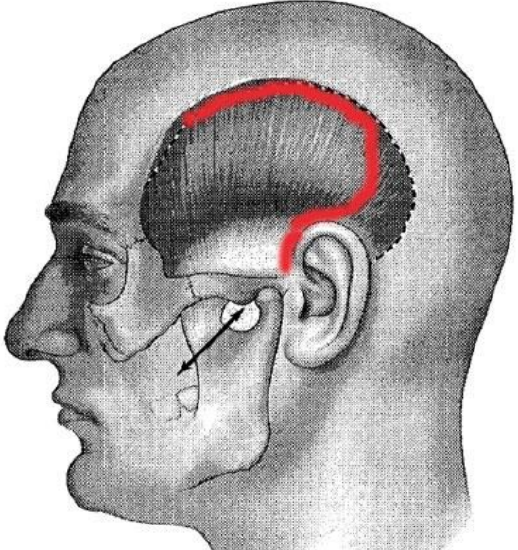
Figure 33: Modified incision
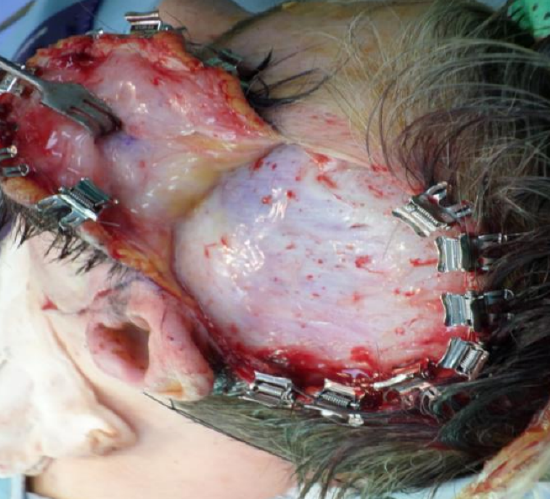
Figure 34: The flap has been elevated in the loose areolar plane above the superficial layer of the deep temporal fascia. Cologne clips have been applied to the skin edge. The temporal fat pad can be seen through the superficial layer of deep temporal fascia just above and in front of the ear at the inferomedial aspect of the wound
- Following injection of local anesthetic with epinephrine, make a bicoronal or hemicoronal incision from the superior temporal line to the attachment of the lobule of the ear (Figure 32)
- The incision may be modified to be curved superiorly to follow the curve of the superior and posterior temporal crest before descending preauricularly (Figure 33)
- Extend the scalp incision down to the superficial layer of the deep temporal fascia, a robust, white layer of glistening fascia (Figure 34)
- Place Cologne or Raney clips along the cut edge of the scalp to control bleeding (Figure 34)
- Elevate this flap in a plane deep to the loose areolar layer and immediately above the superficial layer of the deep temporal fascia to avoid damage to temporal branch of facial nerve if intact
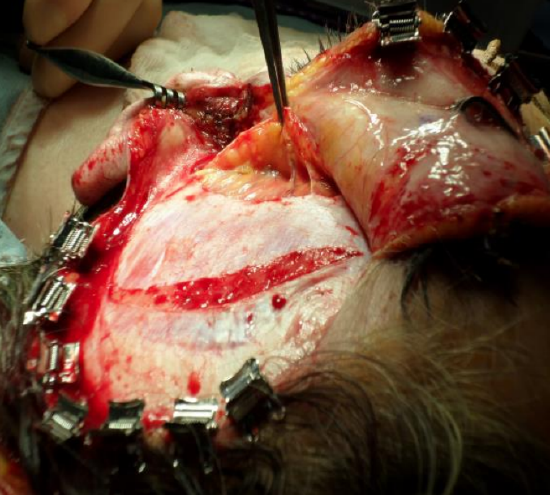
Figure 35: The temporal fat pad is held by the forceps. The incision in the superficial layer of the deep temporal fascia is made to preserve a 1 cm cuff of fascia superiorly to allow for subsequent reapproximation. The temporalis muscle is visible beneath the superficial layer of the deep temporal fascia
- Pause the dissection when the temporal fat pad, which is invested between the superficial and deep layers of deep temporal fascia, is reached above the zygomatic arch (Figures 34, 35)
- Preserve this temporal fat pad to prevent temporal hollowing and to avoid injury to the temporal branch of the facial nerve lies within this loose areolar tissue
- Cut through both layers of the deep temporal fascia along the posterior edge of the temporal fat pad to allow elevation of the temporal fat pad with the overlying fascia (Figure 35)
- Continue to dissect inferiorly between the posterior aspect of the temporal fat pad and the temporalis muscle until the coronoid process of the mandible is reached
- Return to the superior aspect of the temporalis muscle and incise through the superficial layer of the deep temporal fascia approximately 1 cm below the superior temporal crest. This preserves a 1 cm strip of the superficial layer of the deep temporal fascia which is later loosely sutured with a small gap to give the temporalis something to contract against (Figure 35)
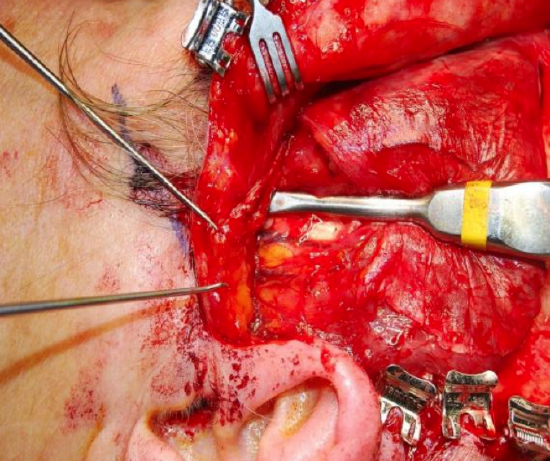
Figure 36: The fascia has been raised off the muscle and a periosteal elevator is being used to free the temporalis muscle to beneath the zygomatic arch. This allows mobilization of the muscle under the zygoma and facilitates good glide of the muscle/tendon complex
- Using a periosteal elevator, free the fan shaped temporalis muscle from its underlying attachment to the cranium in its posterior two-thirds leaving it attached to the skull anteriorly (Figure 36)
- The posterior aspect of the temporalis can now be rotated inferiorly to allow lengthening of the muscle. This lengthening is usually sufficient to permit its tendinous insertion, once detached from the coronoid process of the mandible, to reach the nasolabial fold
- If there is insufficient length e.g. because of scarring, then a fascia lata graft maybe be used as an extension
- Dissection of the deep aspect of the muscle from the cranium following dissection of the superficial aspect of the muscle facilitates preservation of the temporal fat pad
- Place an incision in the nasolabial fold
- Dissect in a subdermal plane for the initial 1-2 cm
- Continue the dissection through the buccal fat pad up to the coronoid process of the mandible
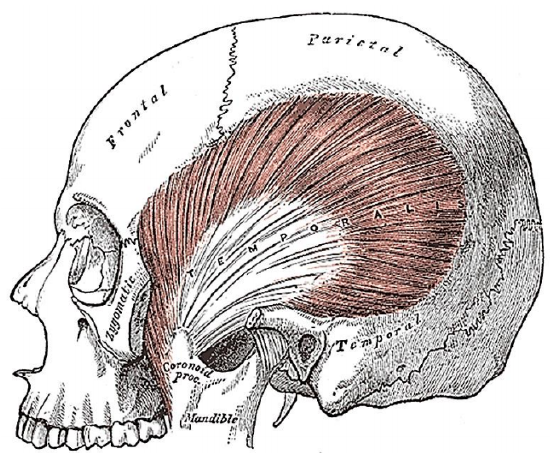
Figure 37: Temporalis muscle inserts into coronoid and anteromedial surface of vertical ramus of mandible
- Identify the insertion of the temporalis to the coronoid process and free the surrounding tissue (Figure 37)
- Hold the tendinous fibers of the temporalis with clamps e.g. a bowel clamp or Satinsky clamp superior to the coronoid process
- Use a saw to sever the tip of the coronoid process for its neck
- Removed any residual bone fragments
- Retain a good hold on the tendinous fibers because if the hold is lost, they are very difficult to retrieve
- Insert the tendinous fibers into the dermis of the upper lip, oral commissure and lower lip to replicate the smile on the contralateral side using 4-0 undyed prolene sutures
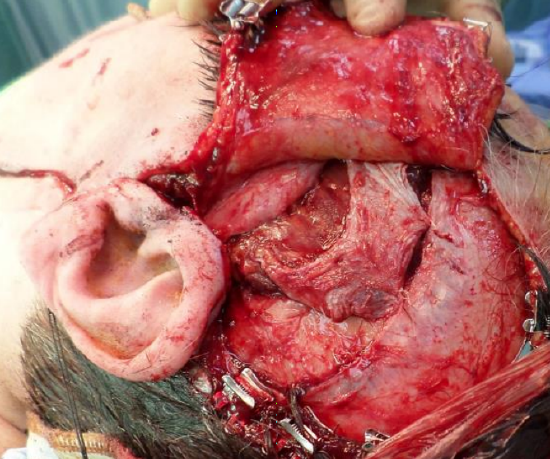
Figure 38: Final position of the temporalis muscle. The posterior part of the muscle has been detached thus allowing it to rotate inferiorly and for it to lengthen. The superficial layer of the deep temporal fascia is loosely approximated to allow the temporalis muscle to contract against a fixed point
- Loosely approximate the superficial layer of deep temporal fascia with 3 to 4 3-0 PDS sutures; this gives the temporalis muscle something to contract against (Figure 38)
- Close the incision in the nasolabial fold with 6-0 nylon interrupted sutures
- Close the scalp with staples and the preauricular wound with 6-0 nylon or monocryl
- Prescribe a soft diet for 3 weeks
- Physical therapy is key to achieving satisfactory muscle function and is instituted within weeks to develop appropriate control and excursion of the transferred muscle
Cross-facial nerve grafting with free muscle transfer
Facial reanimation requires either the restitution of neural control of denervated facial musculature and/or importation of a muscle to reconstruct a symmetrical, resting face and a spontaneous, dynamic smile. CFNG and free muscle transfer is indicated when it is anticipated that the facial muscles on the affected side will not provide useful function following reinnervation. Facial muscles may remain viable up to 12-24 months following injury after which they undergo irreparable atrophy. In situations of nonviable muscle, the CFNG and free muscle transfer provide new, vascularized muscle that can pull in various directions and can potentially produce a spontaneous, dynamic smile. The success of this procedure decreases with increasing patient age.
Numerous techniques have been reported. It may however be performed as a one- or two-stage operation.
One-stage operation
The advantages are that axons need only regenerate over a single anastomotic site and that it avoids the need for a 2nd operation. However, few suitable muscles have sufficiently long nerves. Furthermore, the transferred muscle remains denervated for a longer period of time than with two stage operations; this may compromise the final result.
Two-stage operation
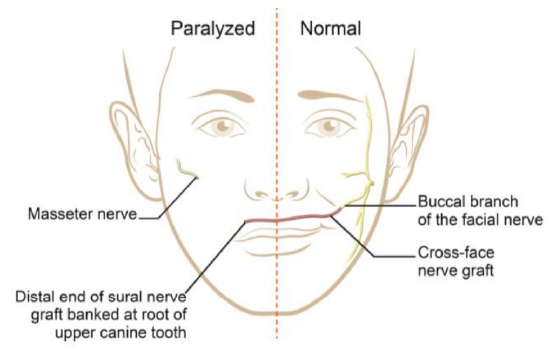
Figure 39: Diagram demonstrating the options to innervate a gracilis muscle: CFNG connected to a functional buccal branch of the normal facial nerve, on the non-paralyzed side; or connection to the masseter nerve on the paralyzed side
- 1st stage (Figure 39)
- Harvest the sural nerve
- Reverse the nerve to minimize axonal growth up side-branches
- Tunnel subcutaneously across the face above the upper lip (Figure 19a)
- Coapt the sural nerve to the working facial nerve
- The senior author’s preference is to “bank” the distal end of the sural nerve graft in the upper buccal sulcus at the level of the root of the upper canine tooth (Figure 19a)
- Alternatively secure the free end of the sural nerve to the tragus or mark it with a permanent, colored suture to facilitate its identification at the 2nd stage
- 2nd stage
- A muscle flap replicates the pull of the zygomaticus major. Although no free muscle transfer completely fulfils all the characteristics, the ideal free donor muscle:
- Provides muscle excursion equal to the normal side of face
- Is small and can be divided into numerous independent slips
- Has reliable vascular and nerve patterns of similar size to that of the recipient structures
- Has a long neurovascular hilum
- Does not cause significant functional or aesthetic donor site morbidity
- Is located sufficiently far away from the face to allow two operating teams to work simultaneously; one team prepares the face and neurovascular structures while the other team harvests the muscle
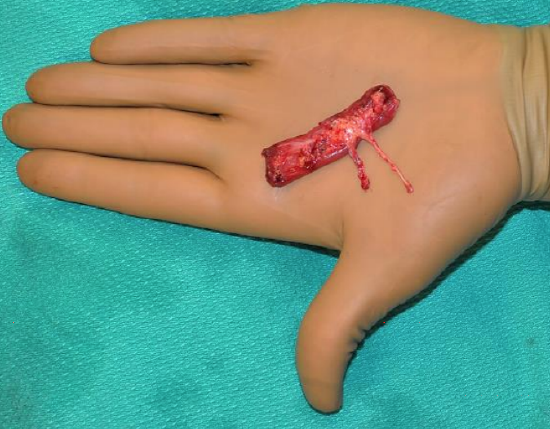
Figure 40: Gracilis muscle with nerve and vascular pedicle
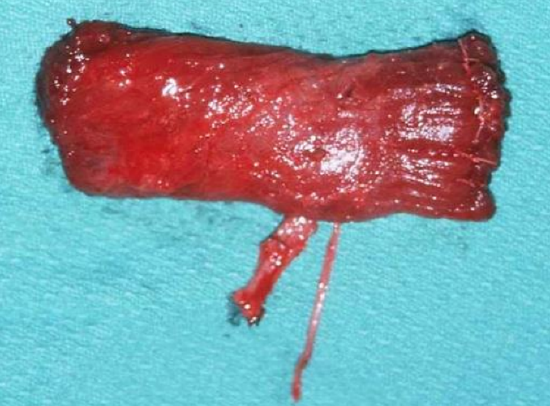
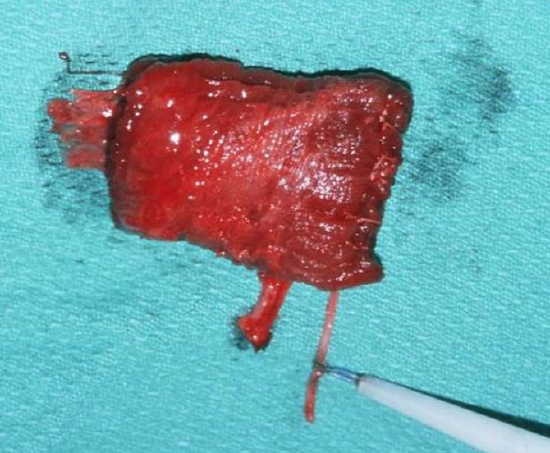
Figures 41a, b: Gracilis muscle with nerve and vascular pedicle uncontracted (a); and contracted (b)
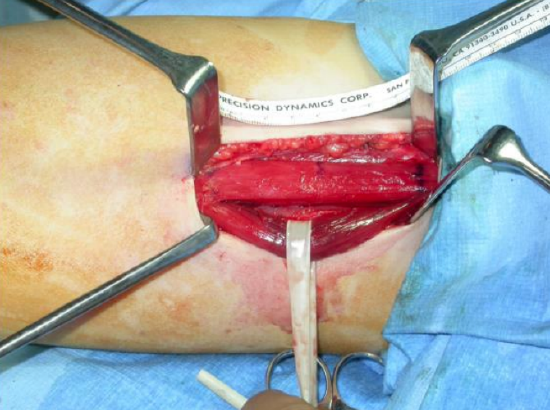
Figure 42: Segmental muscle dissection to reduce bulk
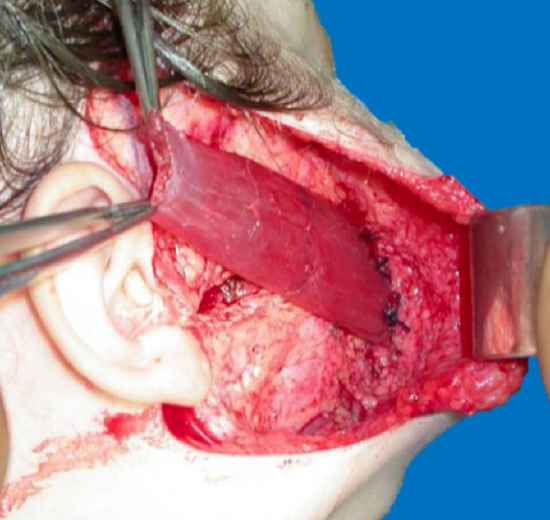
Figure 43: Inset into orbicularis oris
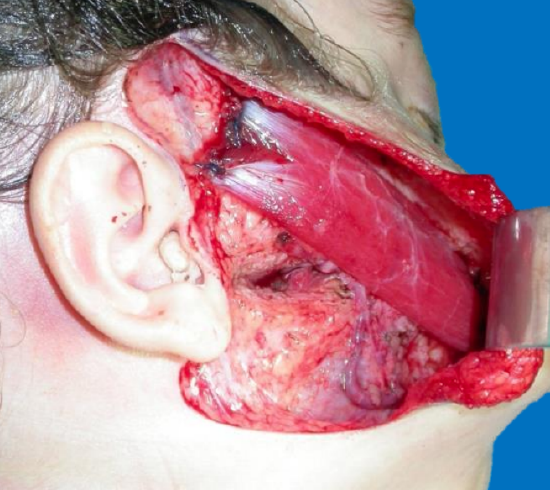
Figure 44: Gracilis muscle sutured to deep temporal fascia
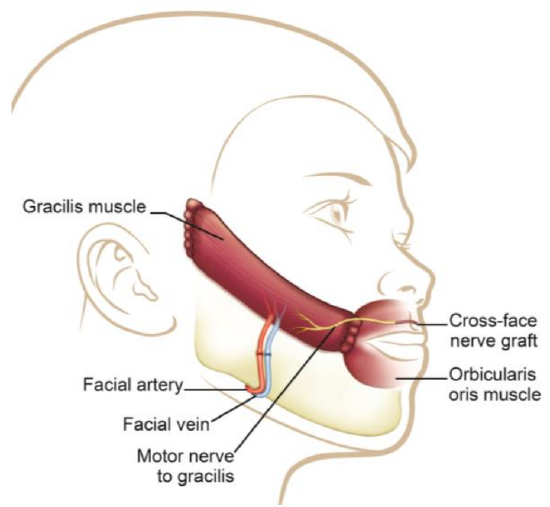
Figure 45: Gracilis flap with vascular anastomosis and CFNG
Gracilis muscle: The gracilis muscle is most commonly used as it is thin, causes minimal donor site morbidity, leaves no functional deficit, has reliable anatomy, and has a relatively long motor nerve (Figure 40). It has strong contractility (Figures 41a, b) and allows a two-team approach. Its bulk may be markedly reduced by segmental muscle dissection (Manktelow and Zuker 1984) (Figure 42). Distally it is inset into the orbicularis oris near the modiolus, just lateral to the oral commissure to replicate the smile on the contralateral side (Figure 43). Proximally it may be inset onto the body of the zygoma or onto the deep temporal fascia (Figures 44, 45). One may need to resect buccal fat or subcutaneous fat to accommodate the bulk of the muscle and to achieve a normal facial contour.
Pectoralis minor: The pectoralis minor has been widely used (Terzis and Manktelow 1982; Harrison 1985). It is a flat muscle, the harvest of which leaves minimal morbidity and it is transferable without excessive bulk. The medial and lateral pectoral nerves and a direct arterial branch from the axillary artery are used for the nerve and vascular anastomoses. However, the anatomy of the neurovascular pedicle may be somewhat variable (Macquillan et al. 2004; Manktelow 1985).
Other muscles used for facial reanimation include the latissimus dorsi, rectus femoris, extensor digitorum brevis, serratus anterior, rectus abdominis and platysma (Dellon & Mackinnon 1985; Mackinnon & Dellon 1988; Hata et al. 1990). Even though the extensor digitorum brevis would appear to be an ideal candidate as it is flat, small and leaving minimal donor site morbidity, results have been disappointing (Mayou et al. 1981; O’Brien et al. 1980). The latissimus dorsi muscle may be divided into two independently innervated zones and has been advocated for single stage bilateral reconstruction in individuals with Möbius syndrome (Mackinnon & Dellon 1988; Woollard et al. 2010); however, it is often bulky in the face and does not facilitate a two-teach approach.
Authors
David C. G. Sainsbury MBBS, BMedSci (Hons), MSc, FRCS (Plast)
Division of Plastic and Reconstructive Surgery
The Hospital for Sick Children, and University of Toronto
Toronto, Ontario, Canada
davidsainsbury@mac.com
Gregory H. Borschel MD, FAAP, FACS
Division of Plastic and Reconstructive Surgery
The Hospital for Sick Children, and University of Toronto
Toronto, Ontario, Canada
borschel@gmail.com
Ronald M. Zuker MD, FRCSC, FAAP, FACS
The Hospital for Sick Children
555 University Avenue
Toronto, Ontario, Canada, M5G 1X8
ronald.zuker@sickkids.ca
Acknowledgement
The authors are extremely grateful to Mr Maniram Ragbir FRCS (Plast), Consultant Plastic Surgeon, Royal Victoria Infirmary, Newcastle Upon Tyne, United Kingdom for his advice regarding the technical aspects of the temporalis lengthening myoplasty
Editor
Johan Fagan MBChB, FCS(ORL), MMed
Professor and Chairman
Division of Otolaryngology
University of Cape Town
Cape Town, South Africa
johannes.fagan@uct.ac.za


