2.1: Atoms
- Page ID
- 11101
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning objectiveS
- Define the term atom and describe its structure in terms of location and charge of its subatomic particles
- Define the term element and distinguish between atom and element
- Distinguish between the terms atomic number and mass number
- Describe the arrangement of electrons in atoms, define valence electrons, and explain the octet rule
- Define the terms ion, cation, anion, and electrolyte
Matter is anything that has mass and takes up space. All things in the universe (i.e. living and non-living things) are considered to be matter. The smallest component of matter normally found is the atom. Then, all things in the universe (including humans) are made of atoms.
Atoms themselves are composed of even smaller subatomic particles (extremely tiny things) called neutrons, protons, and electrons. Protons have a positive electrical charge (+), electrons have a negative electrical charge (-), and neutrons are electrically neutral, meaning they have no charge.
Each one of the different types of atoms in the universe has the same number of protons and electrons. Then, all and every atom in the universe is electrically neutral. For example, one atom of sodium has 11 electrons (-) and 11 protons (+). The 11 positive protons cancel out the 11 negative electrons, and the overall charge of the atom is zero.
Protons and neutrons each weigh one atomic mass unit (amu), and are located at the core of the atom, or nucleus. Electrons move within orbital clouds around the nucleus in orbital shells. Electrons are so small that their mass is considered zero. See figure \(\PageIndex{1}\) and \(\PageIndex{2}\) below, and do activity \(\PageIndex{1}\) below.
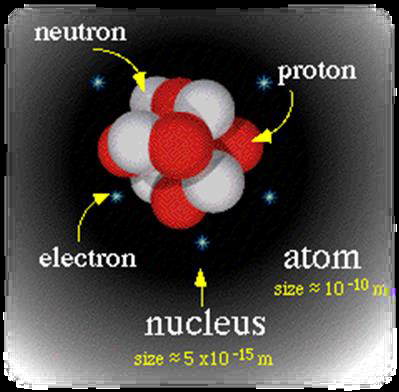
Figure \(\PageIndex{1}\): Representation of an atom. This atom in particular has five electrons and five protons; it is neutral as all atoms are. Electrons’ masses are extremely small compared with protons’ and neutrons’ masses.
The figure below shows a typical planetary representation of an atom highlighting orbital shells.
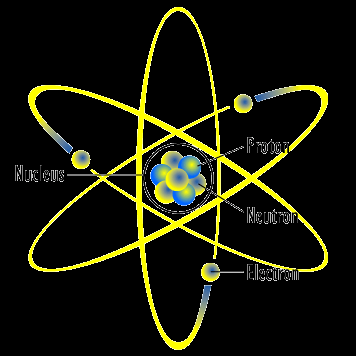
Figure \(\PageIndex{2}\) Representation of an atom as a planetary model. This atom has three protons and three electrons; then, it is neutral, as all atoms are. Electrons are represented much bigger than what they really are, only so they can be clearly seen.
Activity \(\PageIndex{1}\)
Count the number of protons and electrons in the Carbon, Hydrogen, Oxygen, and Nitrogen atoms shown below. Protons are red and neutrons are blue. Each atom should have the same number of protons as electrons.
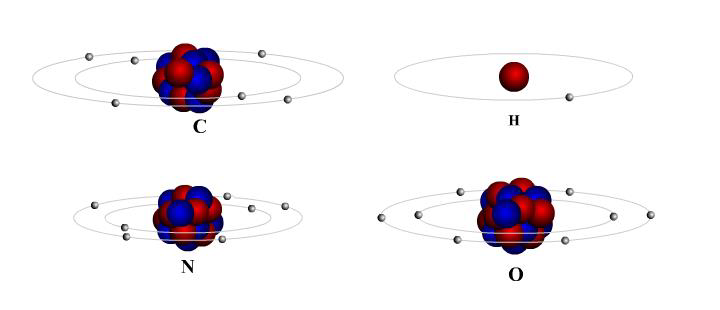
- Answer
-
Carbon: 6 protons and electrons
Hydrogen: 1 protons and electrons
Nitrogen: 7 protons and electrons
Oxygen: 8 protons and electrons
Concepts, terms, and facts check
Study Questions Write your answer in a sentence form (do not answer using loose words)
1. What is an atom?
2. What is a subatomic particle?
3. How many different types of subatomic particles are there in an atom?
4. Where are the different subatomic particles located in an atom?
5. Which subatomic particle is positive?
6. Which subatomic particle is negative?
7. Which subatomic particle is neutral?
8. What is the overall charge of an atom (positive, negative, neutral)?
9. What is an orbital shell?
While all atoms are made of subatomic particles (protons, neutrons, and electrons) not all atoms are the same. There are 118 different types of atoms, called elements. Each element has unique physical and chemical properties. For example, the element carbon and the element oxygen have different melting points, different densities, and different colors. An atom is the smallest unit of an element that retains the properties of that element. We can also define an element as a substance that is made of a single type of atom. See figure \(\PageIndex{3}\) below.


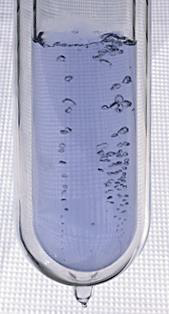
Figure \(\PageIndex{3}\) From left to right, images of the elements sodium (solid), nitrogen (liquid), and oxygen (colorless gas bubbles in the pale blue liquid). The element sodium is made of sodium atoms, the element nitrogen is made of nitrogen atoms, and the element oxygen is made of oxygen atoms.
All known elements in the universe are organized in a chart called the Periodic Table of Elements. The table arranges elements according to their chemical properties, their number of protons, and the way electrons are organized in orbital shells. Elements are symbolized by a chemical symbol (one- or two-letter abbreviation). See figure \(\PageIndex{4}\) below.
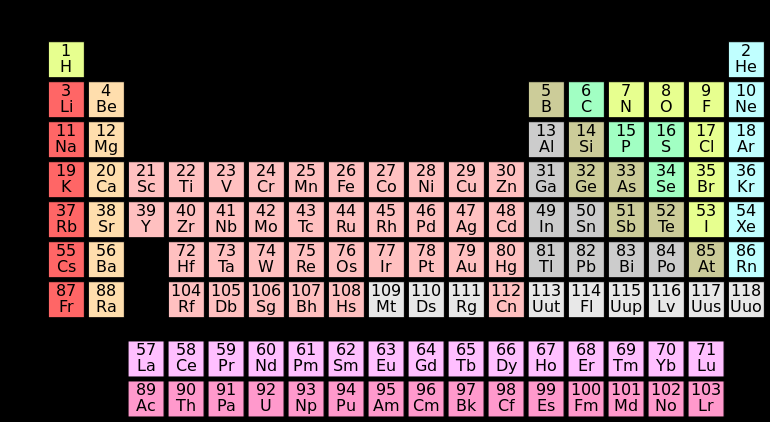
Figure \(\PageIndex{4}\) A standard 18-column form of the Periodic Table of Elements. This figure shows the simplest representation of the table with basic information.
The human body is composed of many different elements as shown in figure \(\PageIndex{5}\) below. For example, carbon (C), hydrogen (H), oxygen (O), nitrogen (N), phosphorous (P), and calcium (Ca), make up 98.5% of the human body weight. Other important elements are potassium (K), sulfur (S), sodium (Na), chlorine (Cl), and magnesium (Mg).
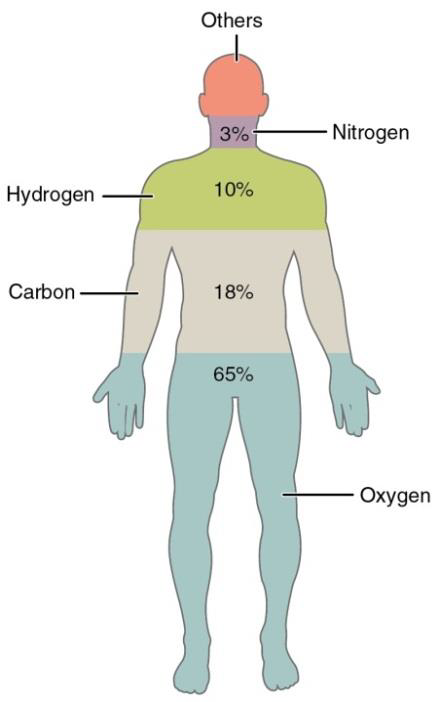
Figure \(\PageIndex{5}\) The main elements that compose the human body
| Element | Symbol | Percentage in Body |
|---|---|---|
| Oxygen | O | 65.0 |
| Carbon | C | 18.5 |
| Hydrogen | H | 9.5 |
| Nitrogen | N | 3.2 |
| Calcium | Ca | 1.5 |
| Phosphorus | P | 1.0 |
| Potassium | K | 0.4 |
| Sulfur | S | 0.3 |
| Sodium | Na | 0.2 |
| Chlorine | Cl | 0.2 |
| Magnesium | Mg | 0.1 |
| Trace elements include boron (B), chromium (Cr), cobalt (Co), copper (Cu), fluorine (F), iodine (I), iron (Fe), manganese (Mn), molybdenum (Mo), selenium (Se), silicon (Si), tin (Sn), vanadium (V), and zinc (Zn). |
less than 1.0 |
Concepts, terms, and facts check
Study Questions Write your answer in a sentence form (do not answer using loose words)
1. What is an element?
2. What is the difference between an atom and an element?
3. What is a chemical symbol?
4. What is the Periodic Table of Elements?
All elements differ in their physical and chemical properties. These properties are given by the number of subatomic particles (protons, electrons and neutrons) they carry. The number of protons an atom carries in its nucleus determines which element it is. For example, one atom of the element carbon (C) always has six protons in its nucleus; and one atom of the element oxygen (O) always has eight protons in its nucleus. In short, all atoms with a particular number of protons belong to the same element.
The number of protons in an atom is denoted by the atomic number, which is shown as a number in the periodic table, usually shown above the chemical symbol of the element. Then, the atomic numbers above each element in the periodic table show the number of protons of each element.
Since atoms are electrically neutral (they carry the same number of protons as electrons); then, the number of protons tells us the number of electrons an element has. For example, nitrogen (N) has an atomic number of 7 (see the periodic table below). Then, nitrogen must have seven protons, and it must also have seven electrons. Sodium (Na) has an atomic number of is 11; then, sodium must have eleven protons, and it must also have eleven electrons. The same applies to all elements in the periodic table. See figure \(\PageIndex{6}\) below.
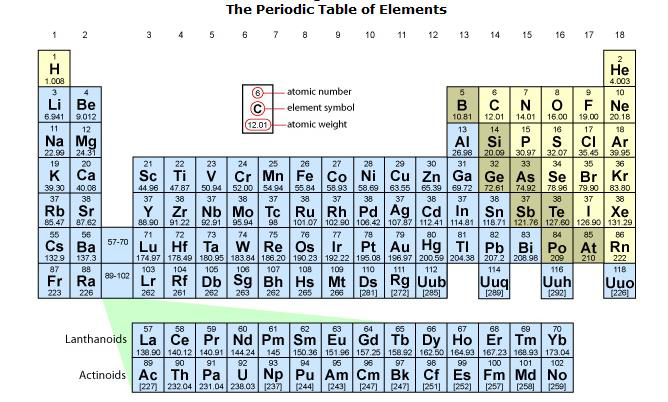
Figure \(\PageIndex{6}\) Periodic Table of Elements showing symbols, atomic numbers and mass numbers. The actual mass of an atom is known as its weight on planet Earth. Then, we consider atomic weight and mass number as the same
The atomic mass of an element is mostly the mass of its neutrons and its protons (since electrons have a negligible mass). For example, the atomic mass of carbon (C) is 12. This comes from adding the number of protons (6) plus the number of neutrons (6), each of which weighs 1 amu. The atomic mass is shown as a decimal number called the mass number in the periodic table, usually shown below the chemical symbol of the element. For the objectives of this course, we consider atomic weight and atomic mass to mean the same thing and ignore the decimal points in the mass number.
Summarizing
Atomic Number = Number of protons = Number of electrons
Mass Number = Atomic Weight = Number of protons + Number of neutrons
Concepts, Terms, and Facts Check
Study Questions Write your answer in a sentence form (do not answer using loose words)
1. What is the atomic number of an element?
2. What is the mass number of an element?
3. How are atomic number, number of protons and number of electron related?
4. How are mass number, number of protons and number of neutrons related?
Electrons are arranged in orbital shells (layers) surrounding the atom’s nucleus. The first shell can carry up to two electrons, the second shell can carry up to eight electrons, and the third shell [can carry up to eighteen electrons but] is usually filled with up to eight electrons. For example, the atomic number of nitrogen is 7. This means that nitrogen has seven protons, and therefore has seven electrons. Two electrons fill the first orbital shell, and five electrons go to the second orbital shell. Hydrogen’s atomic number is 1. This means that hydrogen has one proton, and also one electron, which goes to the first shell. Sodium’s atomic number is 11, so sodium has eleven protons and eleven electrons. The first two fill the first orbital shell, eight more fill the second orbital shell, and the last electron goes to the third orbital shell.
Single atoms by themselves are unstable and chemically reactive (unless their outermost electron shell is already filled with electrons).This means that they tend to react and bond with other atoms until they become non-reactive, or stable. As it is stated by the Octet Rule (oct- = eight), many elements become stable when they have eight electrons in their outermost orbital shell (except hydrogen, which becomes stable having two). For example, nitrogen has five electrons in its second and outermost shell, and completes eight by sharing three electrons with other atom(s). This makes nitrogen stable, which means that by completing an octet it does not need to react with any other atom. We will explore this rule further in a following learning objective.
The number of electrons in the outermost shell of atoms determines the type of bonding that occurs between atoms of the same, or different elements. The electrons found in the outermost shell, and involved in chemical bonding, are called valence electrons. Do activity /(PageIndex{2}/) below.
Activity \(\PageIndex{2}\)
Valence electrons for the some of first elements in the Periodic table. Count the electrons for each element . Note that the first shell has up to two electrons, the second shell up to eight, and the third up to eight too. Electrons in the outermost shell are valence electrons.
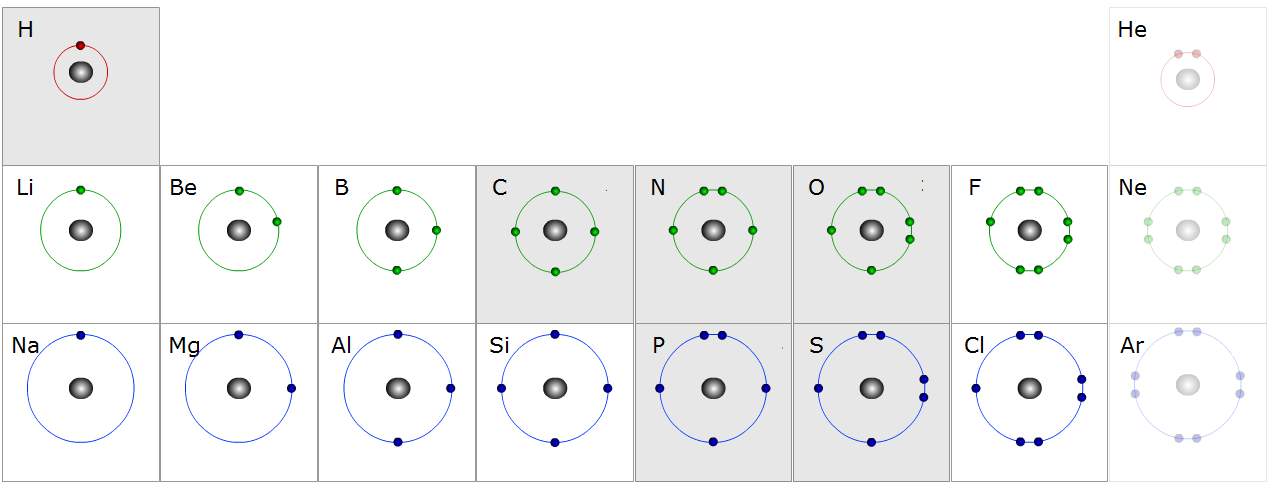
- Answer
-
H: 1 electron
He: 2 electrons
Li: 3 electrons
Be: 4 electrons
B: 5 electrons
C: 6 electrons
N: 7 electrons
O: 8 electrons
F: 9 electrons
Ne: 10 electrons
Na: 11 electrons
Mg: 12 electrons
Al: 13 electrons
Si: 14 electrons
P: 15 electrons
S: 16 electrons
Cl: 17 electrons
Ar: 18 electrons
Concepts, Terms, and Facts Check
Study Questions Write your answer in a sentence form (do not answer using loose words)
1. What does the octet rule say?
2. What are valence electrons?
In order to follow the octet rule and end up with eight electrons in the valence shell (the outermost shell) usually atoms with one or two valence electrons tend to give them away to atoms with six or seven valence electrons that need one or two more electrons to complete eight.
For example, sodium (Na) has a total of eleven electrons: two electrons in the first shell, eight electrons in the second, and one more in the third. By giving up this last electron to an atom of another element, the second shell becomes the outermost, and sodium has eight electrons in its (new) valence shell. Initially, sodium had eleven positive charges from its eleven protons, and eleven negative charges from its eleven electrons, with an overall charge equal to zero. Now that sodium lost one negative charge (the electron), it has an extra positive charge and becomes a cation, a positively charged particle.
In contrast, chlorine (Cl) has a total of seventeen electrons: two electrons in the first shell, eight electrons in the second shell, and seven more in the third (valence shell). By taking up one electron from an atom of other element, its third shell completes eight electrons. Initially, chlorine had seventeen positive charges from its seventeen protons, and seventeen negative charges from its seventeen electrons, with an overall charge equal to zero. Now that chlorine gained one negative charge (the electron), it has an extra negative charge and becomes an anion, a negatively charged particle.
Cations are represented with a superscripted plus sign (+) for each electron lost, and anions are represented with a superscripted minus sign (-) for each electron gained. A single atom can form an ion like in K+, Cl-, F-; Mg2+, Al3+; and also a group of atoms, or parts of a molecule, can form ions like in OH-, HPO4-, NH4+, SO42-.
Electrically charged particles, such as ions, are also called electrolytes. For example, all the ions listed in the previous paragraph are electrolytes. Electrolytes usually dissolve well in water, and a mixture of water and electrolytes can conduct electrical currents.
Concepts, Terms, and Facts Check
Study Questions Write your answer in a sentence form (do not answer using loose words)
1. What is a cation?
2. What is an anion?
3. What is an ion?
4. What is the difference between an element and an ion?
5. What is an electrolyte?

