3.3: Carbohydrates
- Page ID
- 11108
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)learning objectiveS
- Describe the general molecular structure of carbohydrates, and identify their monomers and polymers; list the three subtypes of carbohydrates, and describe their structure and function.
Carbohydrates (carbo- = “carbon”; hydrate = “water”) contain the elements carbon, hydrogen, and oxygen, and only those elements with a few exceptions. The ratio of carbon to hydrogen to oxygen in carbohydrate molecules is 1:2:1. The component carbon (C, carbo-) and the component water (H20, -hydrate) give the name to this group of organic molecules.
Carbohydrates are classified into three subtypes: monosaccharides, (mono- = ”one”, “alone”; saccharide = “sugar, sweet”) disaccharides (di = “two”), and polysaccharides. (poly- = “many, much”). Monosaccharides and disaccharides are also called simple carbohydrates, and are generally referred to as sugars. Simple carbohydrates are small polar molecules, containing several –OH functional groups, which makes them hydrophilic (they dissolve well in water). Polysaccharides, also called complex carbohydrates, are large non polar molecules, and they are not hydrophilic.
The figure below shows the most common monosaccharides: glucose, fructose and galactose (six-carbon monosaccharides), and ribose and deoxyribose (five-carbon monosaccharides). Note that they are all named using the suffix –ose, which means sugar. Carbohydrates are often named “somethingose”.
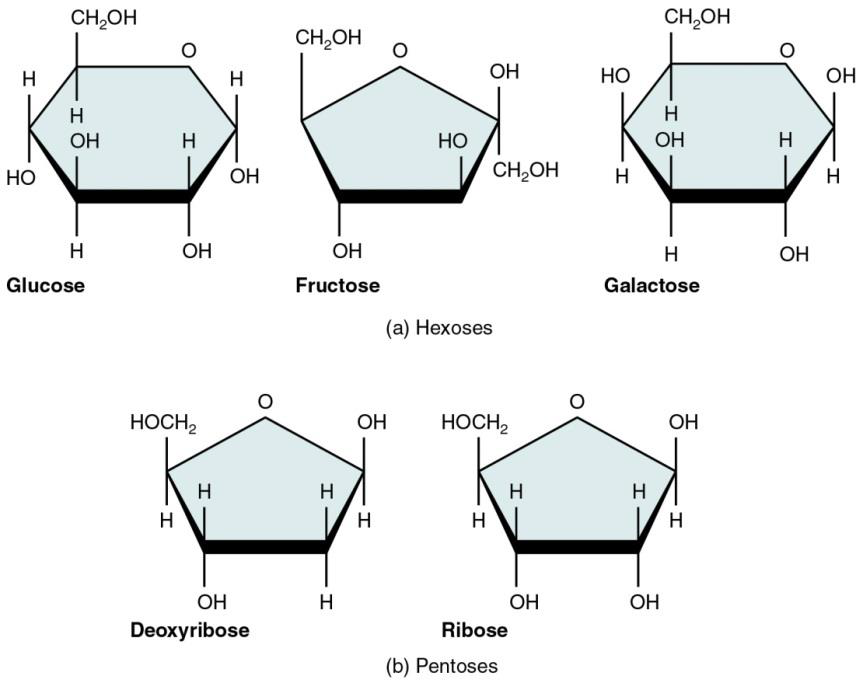
Figure \(\PageIndex{1}\) These monosaccharides respect the ratio 1:2:1 mentioned above: glucose (C6H12O6), fructose (C6H12O6), galactose (C6H12O6), ribose (C5H10O5), deoxyribose (C5H10O4, this one is missing an oxygen). Note that carbohydrates have lots of hydroxyl functional groups (-OH)
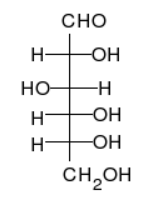
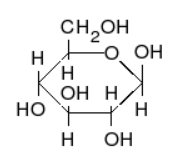
Figure \(\PageIndex{2}\) There are different ways to represent a glucose molecule (C6H12O6). Two of the most common are straight-chain form (left) and ring form (right). Carbon atoms in the vertices are not shown.
Disaccharides form by a covalent bond between two monosaccharides. This type of bond between two monosaccharides is called a glycosidic bond, and energy is needed
to form it.
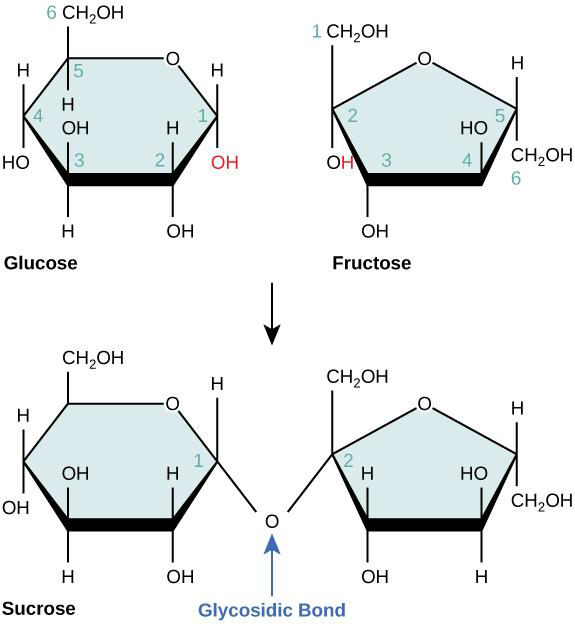
Figure \(\PageIndex{3}\) The disaccharide sucrose is formed when a monomer of glucose and a monomer of fructose join in a dehydration synthesis reaction to form a glycosidic bond. In the process, a water molecule is lost (not shown in the figure). The lost water molecule is formed by -OH and -H shown in red. Oxygen forms covalent bonds with glucose on the left, and fructose on the right.
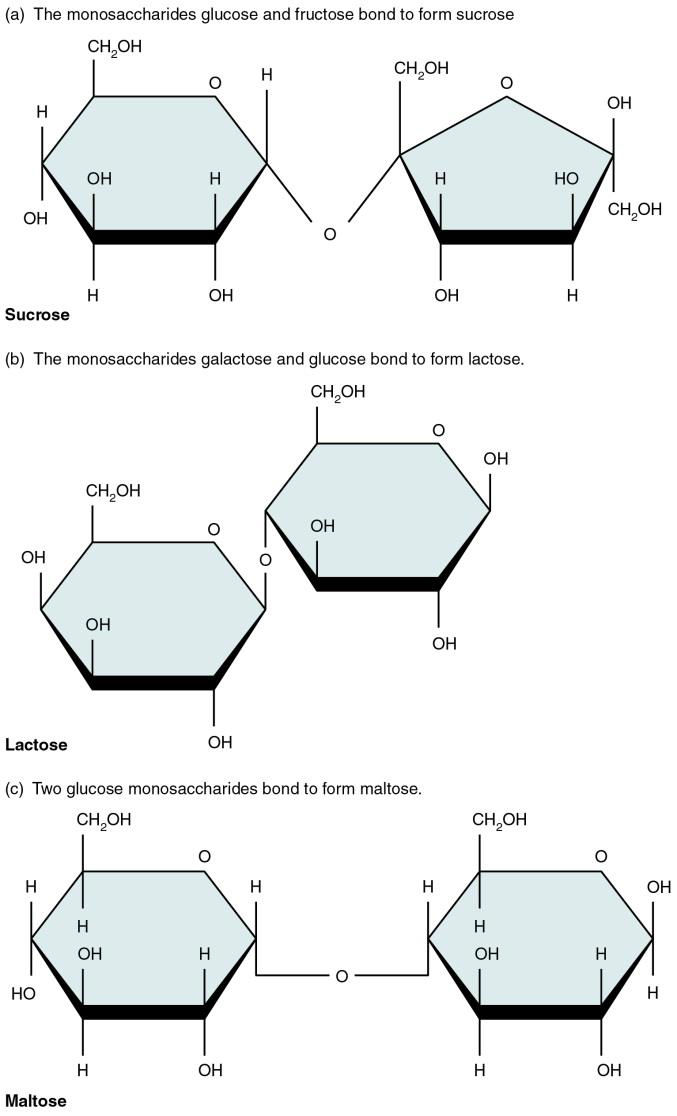
Figure \(\PageIndex{4}\) The most common disaccharides: sucrose (C12H22O11), lactose (C12H22O11), and maltose (C12H22O11)
Polysaccharides are macromolecules composed of repetitive units of the same building block, monosaccharides, similarly to a pearl necklace is composed of many pearls. We can also define polysaccharides as polymers assembled from many smaller covalently bonded monomers. As shown in the Figures and Table below, three important polysaccharides in living organisms are glycogen, starch and cellulose. Glycogen and starch are used as energy stores in animal and plant cells respectively, while cellulose provides structural support in plants and fiber to our diets.
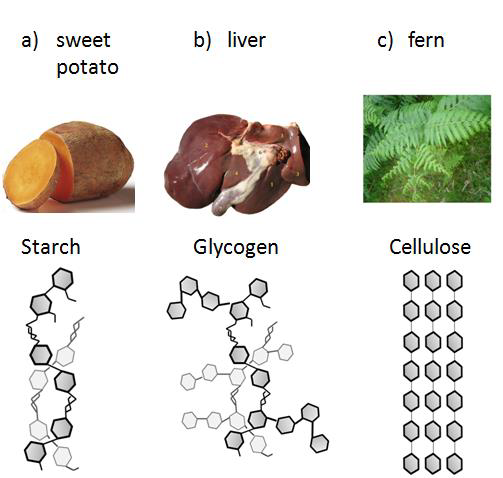
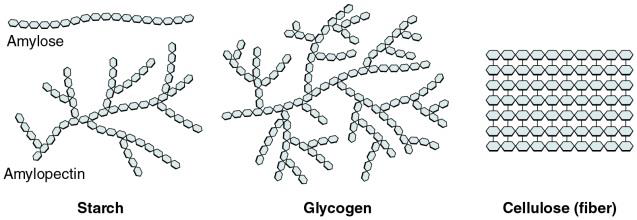
Figure \(\PageIndex{5}\) Amylose and amylopectin found in starch (a) are both polymers made of thousands of glucose molecules (the tiny clear blue hexagons in the figure) linked by covalent bonds. Glycogen (b) and cellulose (c) are also made of thousands of glucose molecules, but organized in a branched and straight pattern, respectively.
| Type of Carbohydrates | No. of Sugar Subunits | Function | Examples and Sources |
|---|---|---|---|
| Monosaccharide (simple sugars) | 1 | Provide energy for cells | Glucose: many plants and fruits, honey, sport drinks; Fructose: fruit, honey, sweetener in many processed foods (high-fructose corn syrup); Galactose: dairy products (milk, butter, cheese, yogurt), beet; Ribose and Deoxyribose: nucleic acids |
| Disaccharide | 2 | Provide energy for cells | Lactose: dairy products; Sucrose: "table sugar", sugarcane, sugar beets, candy; Maltose: germinating seeds, beer |
| Polysaccharide (complex carbohydrates) | many to thousands |
Store energy |
Starch: plants, e.g. potatoes, corn, rice; Glycogen: muscles and liver |
| Structural support | Cellulose: plants |
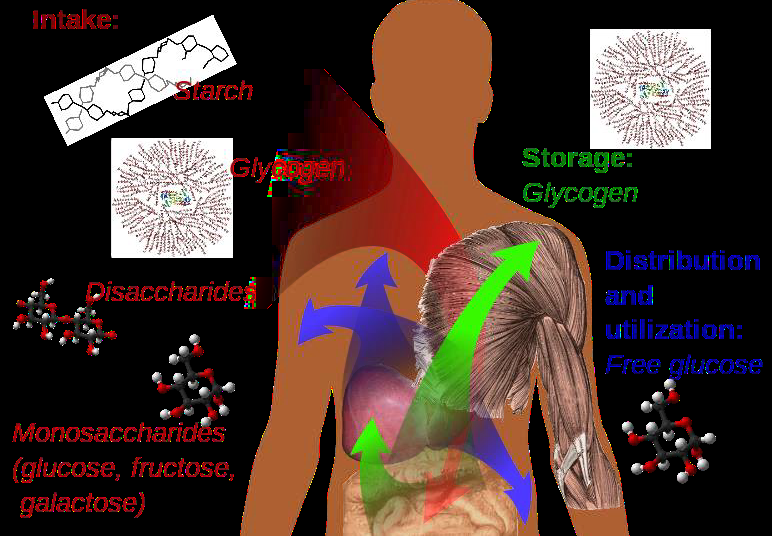
Figure \(\PageIndex{6}\) How we use carbohydrates: we get carbohydrates in the diet as starch, glycogen, lactose, sucrose, maltose, glucose, fructose and galactose. Our blood carries glucose to most cells in our body, where it is used as a source of energy. Our body stores extra glucose as glycogen in muscles and liver.
Concepts, terms, and facts check
Study Questions Write your answer in a sentence form (do not answer using loose words)
- What is a carbohydrate?
- What elements are carbohydrates made of?
- What suffix is used to name carbohydrates?
- What is the difference in structure among monosaccharides, disaccharides, and polysaccharides?
- 5. List all the examples of monosaccharides, disaccharides, and polysaccharides described in the module
- 6. How does the body use monosaccharides, disaccharides, and polysaccharides (what is their function)?

