3.4: Lipids
- Page ID
- 11109
Learning objectiveS
- Describe the general chemical structure of lipids, list three subtypes of lipids important in human functioning, and describe their structure and function
Lipids contain the same elements as carbohydrates: carbon, hydrogen and oxygen (C, H, and O). However, lipids are mainly made of hydrocarbon chains (or rings) and contain fewer polar hydroxyl groups (-OH). This makes most lipids nonpolar hydrophobic molecules (they do not dissolve well in water).
Lipids include a diverse group of organic compounds. We describe only three of them here: triglycerides, phospholipids, and steroids.
Triglycerides include fats and oils. They are the most common type of lipids found in
our body fat tissues and in our diet and serve mostly as an energy store.
Triglycerides consist of one glycerol molecule and three fatty acid molecules (see Figure \(\PageIndex{1}\) below). Glycerol is an organic compound with three carbons, hydrogens, and hydroxyl (-OH) groups. Fatty acids are a long chain of carbons with hydrogens attached to them. A carboxyl (acid) (-COOH) group is attached to one end of the chain. The most common number of carbons in the fatty acid chain ranges from 12 to 18.
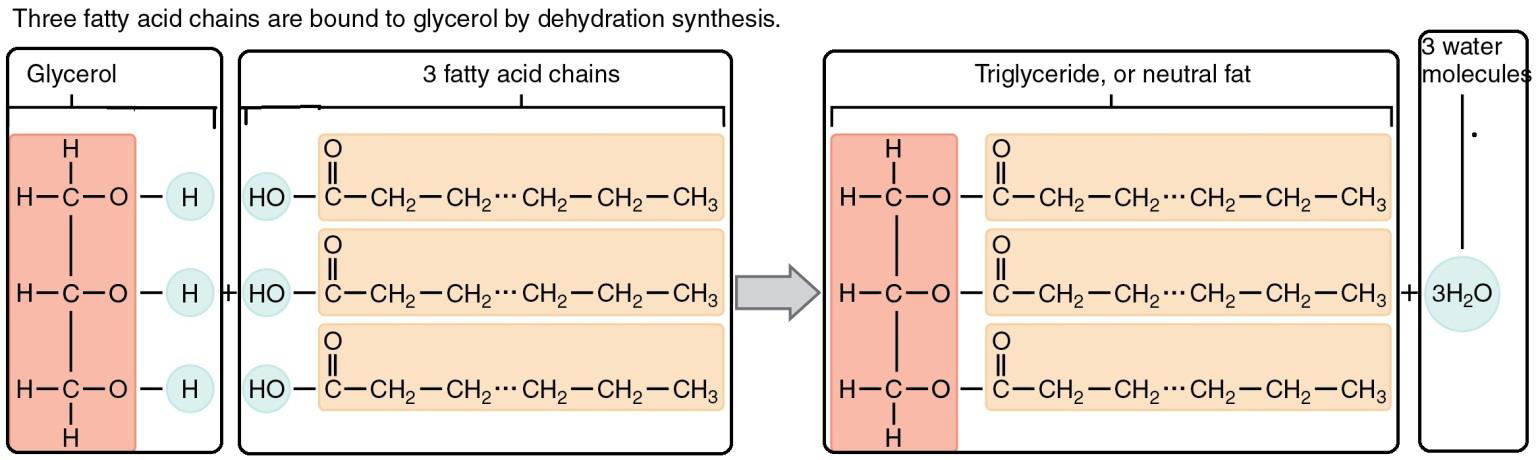 Figure \(\PageIndex{1}\) During triglyceride synthesis, glycerol gives up three hydrogen atoms, and the carboxyl groups on the fatty acids each give up a hydroxyl (-OH) group forming three water molecules. This is a dehydration synthesis reaction
Figure \(\PageIndex{1}\) During triglyceride synthesis, glycerol gives up three hydrogen atoms, and the carboxyl groups on the fatty acids each give up a hydroxyl (-OH) group forming three water molecules. This is a dehydration synthesis reaction
There are two classes of fatty acids: saturated and unsaturated (see Figure \(\PageIndex{2}\) below). Saturated fatty acids have all neighboring carbons in the hydrocarbon chain linked by single covalent bonds. This maximizes the number of hydrogen atoms attached to the carbon skeleton. Then, we say that carbons in the chain are saturated with hydrogens. Unsaturated fatty acids have at least two neighboring carbons in the hydrocarbon chain linked by double covalent bonds. This does not allow all carbons in the chain to maximize the number of hydrogen atoms attached to the carbon skeleton. Then, carbons in the chain are unsaturated (or not saturated) with hydrogens.
Most triglycerides made of unsaturated fatty acids are liquid and are called oils. Triglycerides made of saturated fatty acids are semisolid at room temperature (e.g. fat in butter and meat).
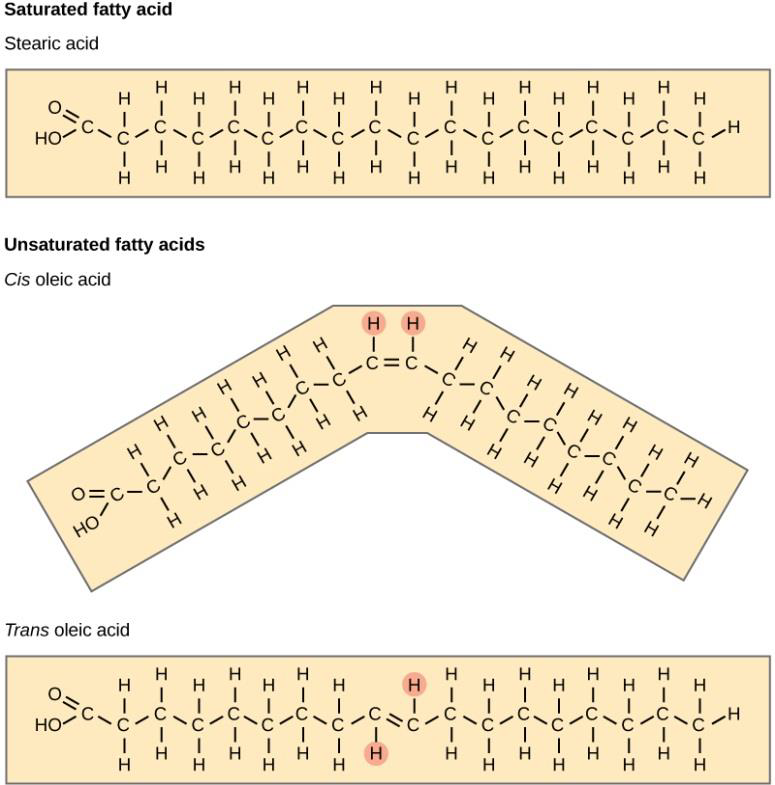
Figure \(\PageIndex{2}\) Saturated fatty acids have hydrocarbon chains connected by single bonds only. Unsaturated fatty acids have one or more double bonds. Each double bond may be in a cis or trans configuration. In the cis configuration, both hydrogens are on the same side of the hydrocarbon chain. In the trans configuration, the hydrogens are on opposite sides. A cis double bond causes a kink in the chain.
Phospholipids are the main components of cell membranes (the layer that separates inner content in a cell from the outside), and membranes inside cells (the enclosing layers that make up internal cell compartments). Phospholipids are composed of fatty acid chains
Phospholipids are composed of fatty acid chains attached to a glycerol backbone (see Figure \(\PageIndex{3}\) below). However, instead of three fatty acids attached as in triglycerides, they have two fatty acids. The third carbon of the glycerol backbone is bound to a modified phosphorous-containing group. The two fatty acid chains are nonpolar, so they don’t interact with water, they are hydrophobic. The phosphorous-containing group is polar, so it does interact well with water, it is hydrophilic. Phospholipids are amphipathic molecules (amphi- = “both, both sides”; pathos = “suffering, feeling”), meaning they have a hydrophobic side and a hydrophilic side.
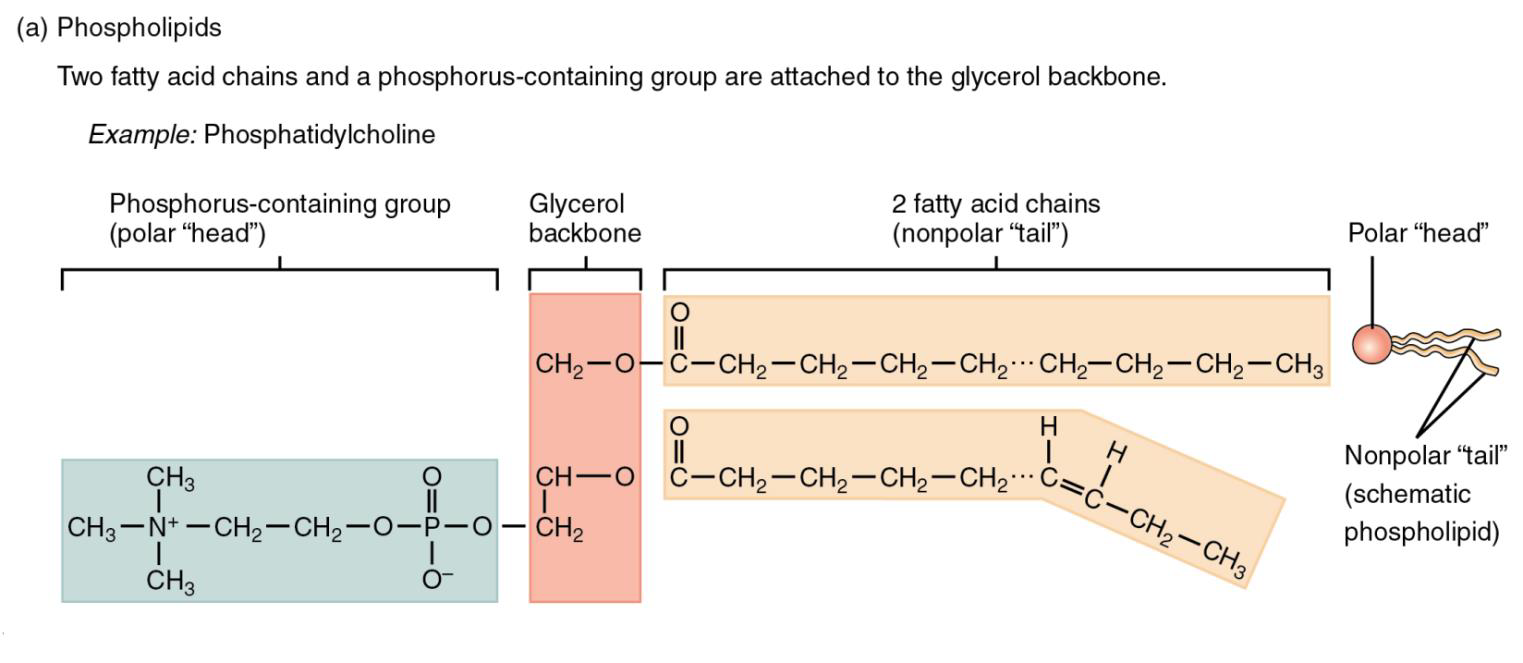 Figure \(\PageIndex{3}\) Phospholipids are composed of glycerol, two nonpolar hydrophobic “tails”, and a polar hydrophilic ”head”. Phospholipids are amphipathic molecules.
Figure \(\PageIndex{3}\) Phospholipids are composed of glycerol, two nonpolar hydrophobic “tails”, and a polar hydrophilic ”head”. Phospholipids are amphipathic molecules.
When phospholipid molecules are in an aqueous (water) solution they often form a bilayer
(two layers) with the hydrophilic head groups of the phospholipids facing the aqueous
solution and the hydrophobic tails in the middle of the bilayer (see Figure \(\PageIndex{4}\) below).
Similarly, a phospholipid bilayer separates the internal and external aqueous environment
of cells.
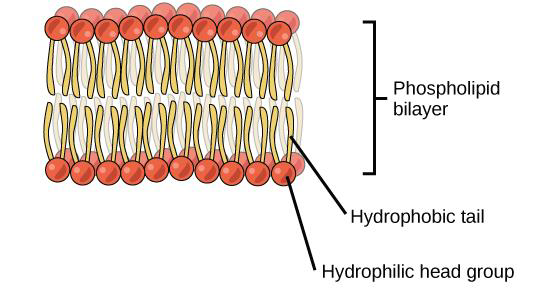
Figure \(\PageIndex{4}\) In a cell membrane (the surface surrounding a cell), a bilayer of phospholipids forms the basic structure. The hydrophobic fatty acid tails of phospholipids face each other, away from the water, whereas the hydrophilic phosphate groups face the outside surfaces, which are aqueous.
Steroids are small lipids where the hydrocarbon backbone has been linked into four rings with various functional groups attached to the rings. Steroids, like other lipids, are nonpolar and hydrophobic. See steroids structure in figure \(\PageIndex{5}\) below.
The most important steroid to human structure and function is cholesterol. Cholesterol helps provide structure to cell membranes and is a component of bile, which helps in the digestion of dietary fats. Cholesterol is also a substrate in the formation of many hormones, signaling molecules that the body releases to regulate processes at distant sites. As shown in Figure \(\PageIndex{5}\), cortisol is one of these hormones and regulates the stress response.
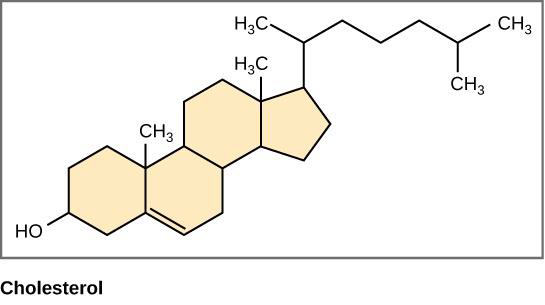
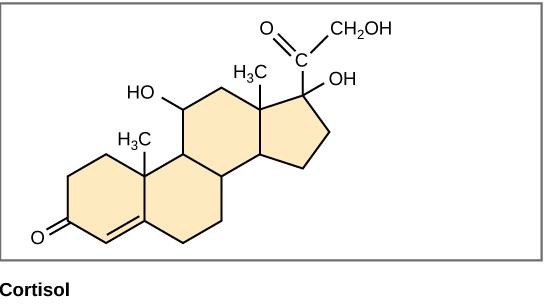
Figure \(\PageIndex{5}\) Structure of steroids. Cholesterol and cortisol share the same four-ring structure typical of steroids.
| Type of Lipid | Structure | General Function | Examples |
|---|---|---|---|
| Triglyceride (fats and oils) | Glycerol plus 3 Fatty acids | Stores energy for use at a later time | |
| Phospholipid | 2 Fatty acids plus glycerol plus a phosphate group | Forms the cell membrane (layer that separates inner content in a cell from the outside), and membranes inside cells (the enclosing layers that make internal cell compartments). | |
| Steroid | Small carbon ring molecules | Structural support of cell membranes; substrate for steroid hormone production; |
Cholesterol |
| Steroid hormones regulate many developmental and metabolic processes | Cortisol, estrogen, testosterone |
Concepts, terms, and facts check
Study Questions Write your answer in a sentence form (do not answer using loose words)
1. What is a lipid?
2. What elements are lipids made of?
3. What are the three classes of lipids described in the module?
4. What is the difference in structure among triglycerides, phospholipids, and steroids (include a description of each one of them in your answer)?
5. What is the difference between saturated fatty acids and unsaturated fatty acids?
6. What does it mean that phospholipids are amphipathic molecules (include a description of a phospholipid molecule in your answer?
7. What are the functions of the different lipids?

