2.2: Protein
- Page ID
- 40933
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Protein is another major macronutrient that, like carbohydrates, are made up of small repeating units. But instead of sugars, protein is made up of amino acids. In the following sections, you will learn more about how protein is synthesized and why it is important in the body.
Amino Acids
Similar to carbohydrates, proteins contain carbon (\(\ce{C}\)), hydrogen (\(\ce{H}\)), and oxygen (\(\ce{O}\)). However, unlike carbohydrates (and lipids) proteins also contain nitrogen (\(\ce{N}\)). Proteins are made up of smaller units called amino acids. This name, amino acid, signifies that each contains an amino (\(\ce{NH2}\)) and carboxylic acid (\(\ce{COOH}\)) groups. The only structural difference in the 20 amino acids is the side group represented by the \(\ce{R}\) below.
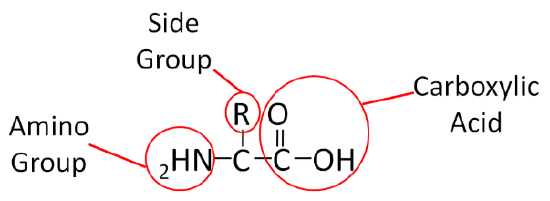
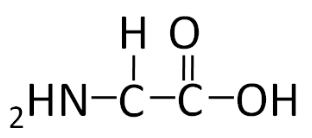
To illustrate the differences in the side group we will consider glycine and alanine, the two simplest amino acids. For glycine the \(\ce{R}\) group is hydrogen (\(\ce{H}\)), while in alanine the \(\ce{R}\) group is a methyl (\(\ce{CH3}\)). The structures of these two amino acids are shown below.
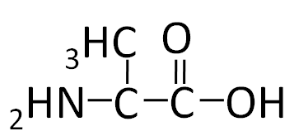
Query \(\PageIndex{1}\)
Individual amino acids are joined together using a peptide bond (green) and is shown in the figure below.
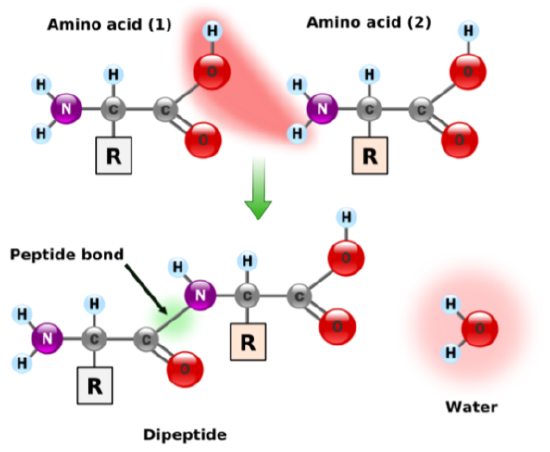
Amino acids can also come together to form tripeptides (three amino acids), oligopeptides (medium size peptide, there isn’t a formal cutoff), and polypeptides (large size). A polypeptide is a chain of amino acids as shown below.
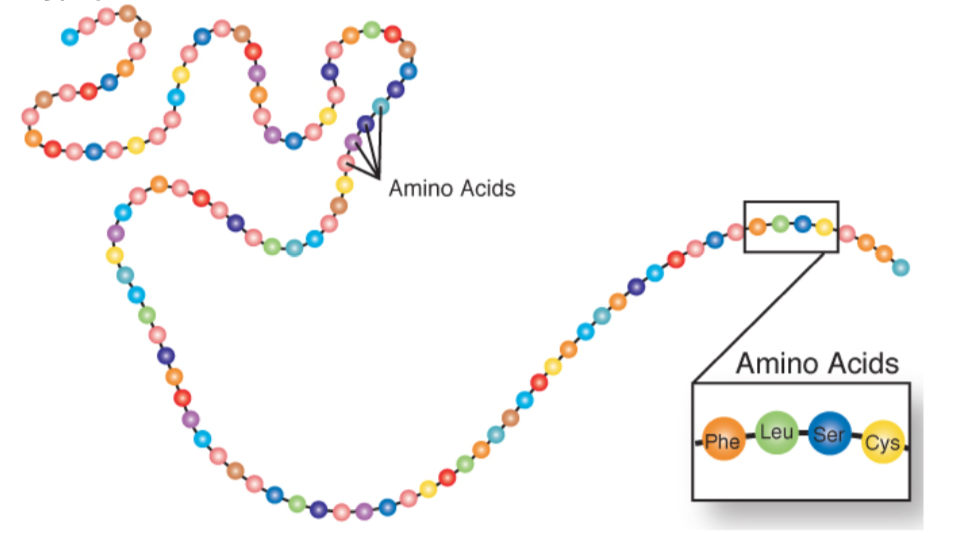
Query \(\PageIndex{2}\)
Protein Synthesis
The process of protein synthesis is not as simple as stringing together amino acids to form a polypeptide. As shown below, this is a fairly involved process. DNA contains the genetic code that is used as a template to create mRNA in a process known as transcription. The mRNA then moves out of the nucleus into the cytoplasm where it serves as the template for translation, where tRNAs bring in individual amino acids that are bonded together to form a polypeptide.

Proteins, known as ribosomes, assist with translation. After translation, the polypeptide can be folded or gain structure as shown below and will be discussed in the next subsection (Protein Structure).

These videos do an excellent job of showing and explaining transcription and translation, respectively.
Query \(\PageIndex{3}\)
Protein Structure
Protein structure is the orientation of the amino acids within a protein. There are four levels of protein structure. Primary structure is the linear polypeptide chain. Secondary structure occurs when hydrogen bonding between amino acids in the same polypeptide chain causes the formation of structures such as beta-pleated sheets and alpha-helices. Tertiary structure occurs as a result of an attraction between different amino acids of the polypeptide chain and interactions between the different secondary structures. Finally, certain proteins contain quaternary structure where multiple polypeptide chains are bonded together to form a larger molecule. Hemoglobin is an example of a protein with quaternary structure. The figure below illustrates the different levels of protein structure.
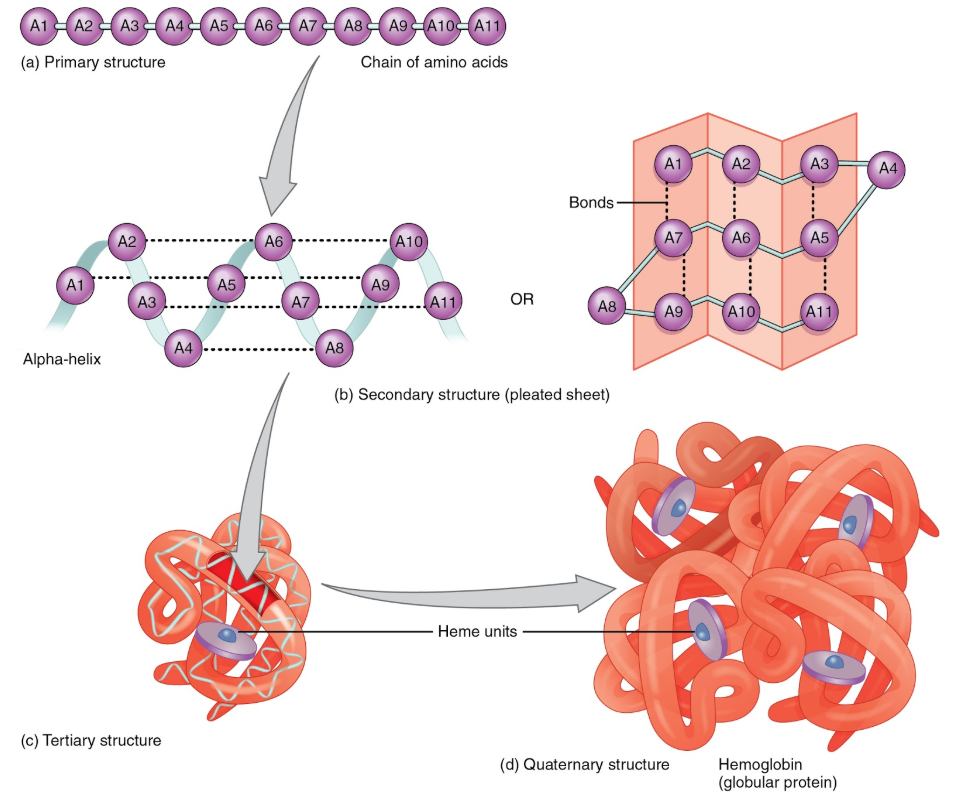
This video does a nice job of illustrating and explaining the different protein structures.
Query \(\PageIndex{4}\)
Protein Functions
There are various functions of proteins in the body that are described below.
Structural
Proteins, such as collagen, serve as the scaffolding of the body, and thus are important for the structure of tissues.
Enzymes
We will discuss a number of enzymes throughout this class, and the vast majority are proteins. An enzyme catalyzes (enhances the rate of) a chemical reaction. The key part of an enzyme is its "active site". The active site is where a compound to be acted on, known as a substrate, enters. Enzymes are specific for their substrates; they do not catalyze reactions on any random compounds floating by. You might have heard the "lock and key" analogy used for enzymes and substrates, respectively. After the substrate enters the active site and binds, the enzyme slightly changes shape (conformation). The enzyme then catalyzes a reaction that, in the example below, splits the substrate into two parts. The products of this reaction are released and the enzyme returns to its native or original shape. It is then ready to catalyze another reaction. The figure and video below nicely illustrate the function of an enzyme.
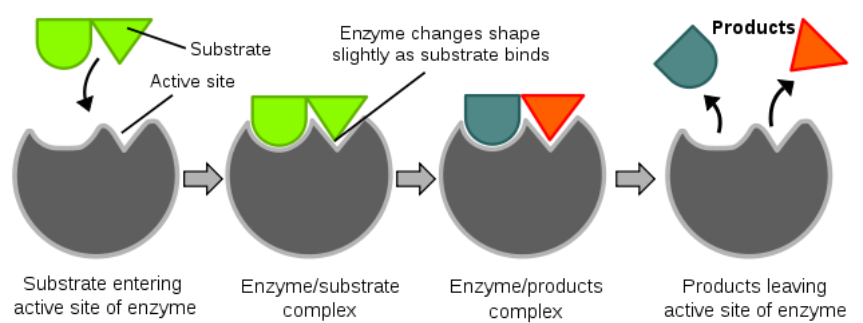
Enzymes’ names commonly end in -ase, and many are named for their substrate. For example the enzyme amylase cleaves bonds found in amylose and amylopectin.
Hormones
Many hormones are proteins. A hormone is a compound that is produced in one tissue, released into circulation, then has an effect on a different organ. Most hormones are produced from several organs, collectively known as endocrine organs. Insulin is an example of a hormone that is a protein. The video below describes and illustrates the functions of hormones.
Fluid Balance
Proteins help to maintain the balance between fluids in the plasma and the interstitial fluid. Interstitial fluid is the fluid that surrounds cells. Interstitial fluid and plasma (fluid part of blood) are the two components of extracellular fluid, or the fluid outside of cells. The following figure illustrates the exchange of fluid between interstitial fluid and plasma.

Acid-Base Balance
Proteins serve as buffers, meaning that they help to prevent the pH of the body from getting too high or too low.
Transport
Transport proteins move molecules through circulation or across cell membranes. One example is hemoglobin that transports oxygen through the body. We will see a number of other examples as we move through class.
Immune Function
Antibodies are proteins that recognize antigens (foreign substances that generate antibody or inflammatory response) and bind to and inactivate them. Antibodies are important in our ability to ward off disease.
Other Functions
Proteins can also serve as neurotransmitters and can be used for energy by forming glucose through gluconeogenesis.
Query \(\PageIndex{5}\)
Types of Amino Acids
There are 20 amino acids our body uses to synthesize proteins. These amino acids can be classified as essential, non-essential, or conditionally essential. The table below shows how the 20 amino acids are classified.
| Essential | Conditionally Essential | Nonessential |
|---|---|---|
| Histidine | Arginine | Alanine |
| Isoleucine | Cysteine | Asparagine |
| Leucine | Glutamine | Aspartic Acid or Aspartate |
| Lysine | Glycine | Glutamic Acid or Glutamate |
| Methionine | Proline | Serine |
| Phenylalanine | Tyrosine | |
| Threonine | ||
| Tryptophan | ||
| Valine |
The body cannot synthesize nine amino acids. Thus, it is essential that these are consumed in the diet. As a result, these amino acids are known as essential, or indispensable, amino acids. As an example of how amino acids were determined to be essential, Dr. William C. Rose at the University of Illinois discovered that threonine was essential by feeding different diets to graduate students at the university as described in the following link.
Nonessential, or dispensable, amino acids can be made in our body, so we do not need to consume them. Conditionally essential amino acids become essential for individuals in certain situations. An example of a condition when an amino acid becomes essential is the disease phenylketonuria (PKU). Individuals with PKU have a mutation in the enzyme phenylalanine hydroxylase, which normally adds an alcohol group (OH) to the amino acid phenylalanine to form tyrosine as shown below.
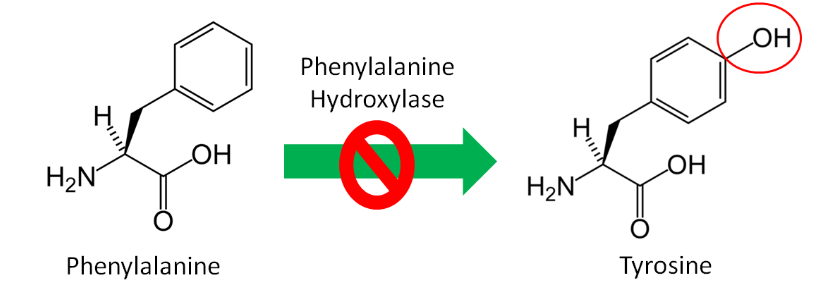
Since tyrosine cannot be synthesized by people with PKU, it becomes essential for them. Thus, tyrosine is a conditionally essential amino acid. Individuals with PKU have to eat a very low protein diet and avoid the alternative sweetener aspartame, because it can be broken down to phenylalanine. If individuals with PKU consume too much phenylalanine, phenylalanine and its metabolites, can build up and cause brain damage and severe mental retardation. The drug Kuvan was approved for use with PKU patients in 2007 who have low phenylalanine hydroxylase activity levels. You can learn more about this drug using the link below.
Web Link
Query \(\PageIndex{6}\)
Query \(\PageIndex{7}\)
Amino Acid Structures
It is a good idea to have a general idea of the structure of the different amino acids and to be able to recognize them as amino acids. You are not expected to memorize these structures. Often I say the name of amino acids and not all students understand that I am talking about an amino acid. Each amino acid differs only by its side group, which is circled in red in each figure below. Also, the more familiar you become with chemical structures, the more prepared you will be for later classes.


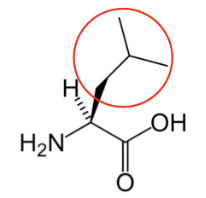
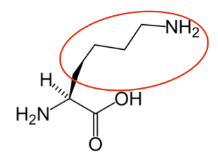

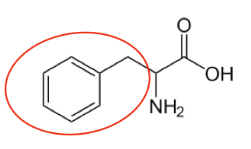



Figure \(\PageIndex{13}\): Essential amino acids12-20
You may hear someone talk about the branch chain amino acids, which are all essential amino acids, but they are singled out in the figure below. These amino acids have branched carbons in their side chains.

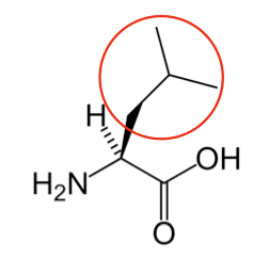

Figure \(\PageIndex{14}\): Branched chain amino acids20,14,13

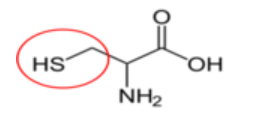

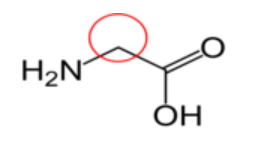


Figure \(\PageIndex{15}\): Conditionally essential amino acids21-26

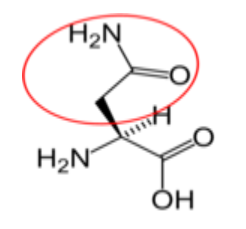


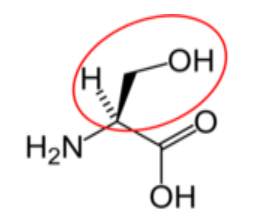
Figure \(\PageIndex{16}\): Nonessential amino acids27-31
Query \(\PageIndex{8}\)
Protein Quality
Proteins can be classified as either complete or incomplete. Complete proteins provide adequate amounts of all nine essential amino acids. Animal proteins such as meat, fish, milk, and eggs are good examples of complete proteins. Incomplete proteins do not contain adequate amounts of one or more of the essential amino acids. For example, if a protein doesn't provide enough of the essential amino acid leucine it would be considered incomplete. Leucine would be referred to as the limiting amino acid, because there is not enough of it for the protein to be complete. Most plant foods are incomplete proteins, with a few exceptions such as soy. The table below shows the limiting amino acids in some plant foods.
| Food | Limiting Amino Acid(s) |
|---|---|
| Bean and Most Legumes | Methionine, Tryptophan |
| Tree Nuts and Seeds | Methionine, Lysine |
| Grains | Lysine |
| Vegetables | Methionine, Lysine |
Query \(\PageIndex{9}\)
Complementary Proteins
Even though most plant foods do not contain complete proteins, it does not mean that they should be sworn off as protein sources. It is possible to pair foods containing incomplete proteins with different limiting amino acids to provide adequate amounts of the essential amino acids. These two proteins are called complementary proteins, because they supply the amino acid(s) missing in the other protein. A simple analogy would be that of a 4 piece puzzle. If one person has 2 pieces of a puzzle, and another person has 2 remaining pieces, neither of them have a complete puzzle. But when they are combined, the two individuals create a complete puzzle.

Two examples of complementary proteins are shown below.

It should be noted that complementary proteins do not need to be consumed at the same time or meal. It is currently recommended that essential amino acids be met on a daily basis, meaning that if a grain is consumed at one meal, a legume could be consumed at a later meal, and the proteins would still complement one another35.
Query \(\PageIndex{10}\)
Measures of Protein Quality
How do you know the quality of the protein in the food you consume? The protein quality of most foods has been determined by one of the methods below.
- Biological Value (BV) - (Grams of nitrogen retained / grams of nitrogen absorbed) x 100
- Protein Efficiency Ratio (PER) - Grams of weight gained / grams of protein consumed. This method is commonly performed in growing rats.
- Amino Acid Score (AAS) - Test food limiting essential amino acid (mg/g protein) / needs of same essential amino acid (mg/g protein). An amino acid score of 100 or more indicates that the protein contains adequate amounts of all essential amino acids and thus is considered complete. An amino acid score of less than 100 indicates that at least 1 amino acid is limiting and it is incomplete.
- Protein Digestibility Corrected Amino Acid Score (PDCAAS) - Amino Acid Score x Digestibility
This is the most widely used method and was preferred by the Food and Agriculture Organization and World Health Organization (WHO) until recently36,37.
The following table shows the protein quality measures for some common foods.
| Protein | PER | Digestibility | AAS (%) | PDCAAS |
|---|---|---|---|---|
| Egg | 3.8 | 98 | 121 | 100* |
| Milk | 3.1 | 95 | 127 | 100* |
| Beef | 2.9 | 98 | 94 | 92 |
| Soy | 2.1 | 95 | 96 | 91 |
| Wheat | 1.5 | 91 | 47 | 42 |
*PDCAAS scores are truncated (cut off) at 100. These egg and milk scores are actually 118 and 121 respectively.
The Food and Agricultural Organization (FAO) recently recommended that PDCAAS be replaced with a new measure of protein quality, the Digestible Indispensable Amino Acid Score (DIAAS). “DIAAS is defined as: DIAAS % = 100 x (mg of digestible dietary indispensable amino acid in 1g of dietary protein / mg of the same dietary indispensable amino acid in 1g of the reference protein).” Ileal digestibility should be utilized to determine the digestibility in DIAAS; ideally in humans, but if not possible in growing pigs or rats37.
The main differences between DIAAS and PDCAAS are that DIAAS:
- Takes into account individual amino acids’ digestibility rather than protein digestibility.
- Focuses on ileal instead of fecal (total) digestibility.
- Has 3 different reference patterns (different age groups, 0-6 months, 6 months- 3 years, 3-10 years old) instead of a single reference pattern
- Are not truncated38.
Query \(\PageIndex{11}\)
Protein-Energy Malnutrition
Protein deficiency rarely occurs alone. Instead it is often coupled with insufficient energy intake. As a result, the condition is called protein-energy malnutrition (PEM). This condition is not common in the U.S., but is more prevalent in less developed countries. Kwashiorkor and marasmus are the two forms of protein energy malnutrition. They differ in the severity of energy deficiency as shown in the figure below.
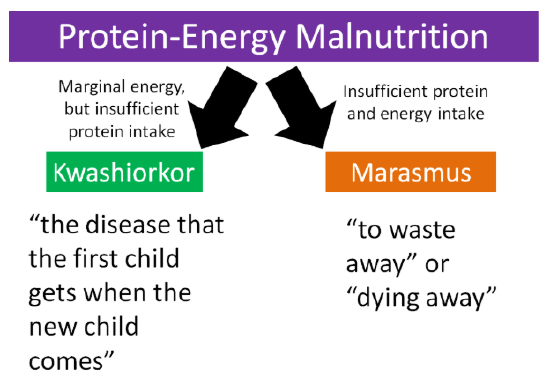
Kwashiorkor is a Ghanaian word that means "the disease that the first child gets when the new child comes39." The characteristic symptom of kwashiorkor is a swollen abdomen. Energy intake could be adequate, but protein consumption is too low.

The video below does a nice job showing the symptoms of the condition.
Marasmus means "to waste away" or "dying away", and thus occurs in individuals who have inadequate protein and energy intakes.

Query \(\PageIndex{12}\)
Query \(\PageIndex{13}\)
References
- en.Wikipedia.org/wiki/File:Pe...mationball.svg
- http://www.genome.gov/Glossary/index.cfm?id=149
- www.genome.gov/Pages/Hyperion...essenger%20RNA
- en.Wikipedia.org/wiki/File:Pr...nsynthesis.png
- "225 Peptide Bond-01" by OpenStax College - Anatomy & Physiology, Connexions Web site. http://cnx.org/content/col11496/1.6/, Jun 19, 2013.. Licensed under CC BY 3.0 via Commons - https://commons.wikimedia.org/wiki/F...de_Bond-01.jpg
- en.Wikipedia.org/wiki/File:Co...riplehelix.png
- en.Wikipedia.org/wiki/File:In...it_diagram.svg
- en.Wikipedia.org/wiki/File:Il...irculation.jpg
- Anonymous. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). Protein and Amino Acids. Institute of Medicine, Food and Nutrition Board. 2005 http://books.nap.edu/openbook.php?re...10490&page=589
- en.Wikipedia.org/wiki/Phenyl...nylalanine.svg
- en.Wikipedia.org/wiki/Tyrosi...L-Tyrosine.svg
- en.Wikipedia.org/wiki/Histid..._Histidine.svg
- en.Wikipedia.org/wiki/Isoleu...Isoleucine.svg
- en.Wikipedia.org/wiki/Leucin...e_Nonionic.svg
- en.Wikipedia.org/wiki/Lysine...D-skeletal.png
- en.Wikipedia.org/wiki/Methio...Methionine.svg
- en.Wikipedia.org/wiki/Phenyl...nylalanine.svg
- en.Wikipedia.org/wiki/Threon...eonineasdf.png
- en.Wikipedia.org/wiki/Trypto...Tryptophan.svg
- en.Wikipedia.org/wiki/Valine...notatpH7.4.png
- en.Wikipedia.org/wiki/Argini...-_Arginine.svg
- en.Wikipedia.org/wiki/Cystei...L-Cysteine.svg
- en.Wikipedia.org/wiki/Glutam...-Glutamine.svg
- en.Wikipedia.org/wiki/Glycin..._-_Glycine.svg
- en.Wikipedia.org/wiki/Prolin..._-_Proline.svg
- en.Wikipedia.org/wiki/Tyrosi...L-Tyrosine.svg
- en.Wikipedia.org/wiki/Alanin..._L-Alanine.svg
- en.Wikipedia.org/wiki/Aspara...Asparagine.svg
- en.Wikipedia.org/wiki/Aspart...tic_Acidph.png
- en.Wikipedia.org/wiki/Glutam..._Non-ionic.png
- en.Wikipedia.org/wiki/Serine...-_L-Serine.svg
- Wardlaw GM, Hampl J. (2006) Perspectives in nutrition. New York, NY: McGraw-Hill.
- commons.wikimedia.org/wiki/F...y_sandwich.jpg
- en.Wikipedia.org/wiki/File:Re...s_and_rice.jpg
- Young VR, Pellett PL. (1994) Plant proteins in relation to human protein and amino acid nutrition. Am J Clin Nutr. May; 59 (5 Suppl): 1203S-1212S.
- Schaafsma G. (2000) The protein digestibility-corrected amino acid score. J Nutr 130(7): 1865S-1867S.
- www.fao.org/ag/humannutrition...04ffc17f06.pdf
- Rutherford SM, Fanning AC, Miller BJ, Moughan PJ. Protein Digestibility-Corrected Amino Acid Scores and Digestible Indispensable Amino Acid Scores Differentially Describe Protein Quality in Growing Male Rats. J Nutr. 145(2): 372-379.
- Byrd-Bredbenner C, Moe G, Beshgetoor D, Berning J. (2009) Wardlaw's perspectives in nutrition. New York, NY: McGraw-Hill.
- en.Wikipedia.org/wiki/File:Starved_girl.jpg
- en.Wikipedia.org/wiki/File:Starved_child.jpg


