2.3: Lipids
- Page ID
- 40934
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Lipids, commonly referred to as fats, have a poor reputation among some people, in that "fat free" is often synonymous with healthy. We do need to consume certain fats and we should try to incorporate some fats into our diets for their health benefits. However, consumption of certain fats is also associated with greater risk of developing chronic disease(s). In this section we will dive deeper into fats and why they do not need to be feared altogether.
How Does Fat Differ From Lipids?
The answer you receive from this question will depend on who you ask, so it is important to have an understanding of lipids and fats from a chemical and nutritional perspective.
To a chemist, lipids consist of:
- Triglycerides
- Fatty Acids
- Phospholipids
- Sterols
These compounds are grouped together because of their structural and physical property similarities. For instance, all lipids have hydrophobic (water-fearing) properties. Chemists further separate lipids into fats and oils based on their physical properties at room temperature:
- Fats are solid at room temperature
- Oils are liquid at room temperature
From a nutritional perspective, the definition of lipids is the same. The definition of a fat differs, however, because nutrition-oriented people define fats based on their caloric contribution rather than whether they are solid at room temperature. Thus, from a nutrition perspective:
- Fats are triglycerides, fatty acids, and phospholipids that provide 9 kcal/g.
The other difference is that from a caloric perspective, an oil is a fat. For example, let's consider olive oil. Clearly, it is an oil according to a chemist definition, but from a caloric standpoint it is a fat because it provides 9 kcal/g.
The following sections will discuss the different lipid classes introduced above in detail.
Query \(\PageIndex{1}\)
Fatty Acids
Fatty acids are lipids themselves, and they are also components of triglycerides and phospholipids. Like carbohydrates, fatty acids are made up of carbon (\(\ce{C}\)), hydrogen (\(\ce{H}\)), and oxygen (\(\ce{O}\)).
On one end of a fatty acid is a methyl group (\(\ce{CH3}\)) that is known as the methyl or omega end. On the opposite end of a fatty acid is a carboxylic acid (\(\ce{COOH}\)). This end is known as the acid or alpha end. Figure \(\PageIndex{1}\) shows the structure of fatty acids.
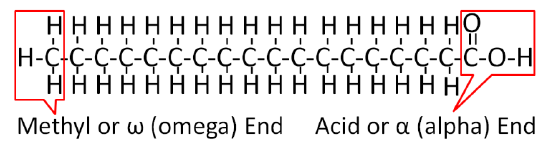
There are a number of fatty acids in nature that we consume that differ from one another in three ways:
- Carbon chain length (i.e. 6 carbons, 18 carbons)
- Saturation/unsaturation
- Double bond configuration (cis, trans)
Carbon Chain Length
Fatty acids differ in their carbon chain length (number of carbons in the fatty acid). Most fatty acids contain somewhere between 4-24 carbons, with even numbers (i.e. 8, 18) of carbons occurring more frequently than odd numbers (i.e. 9, 19). Fatty acids are classified as short-chain fatty acids, medium-chain fatty acids, and long-chain fatty acids based on their carbon chain length using the criteria shown in the table below.
| Classification | # of carbons |
|---|---|
| Short-Chain Fatty Acid | < 6 |
| Medium-Chain Fatty Acid | 6-12 |
| Long-Chain Fatty Acid | > 12 |
Carbon chain length also impacts the physical properties of the fatty acid. As the number of carbons in a fatty acid chain increases, so does the melting point as illustrated in the figure below.
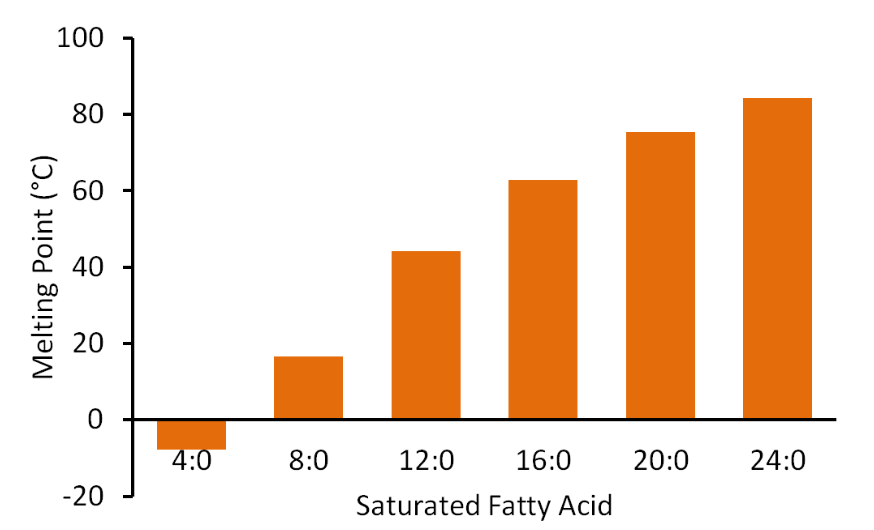
Thus, shorter chain fatty acids are more likely to be liquid, while longer chain fatty acids are more likely to be solid at room temperature (20-25ᐤC, 68-77ᐤF).
Query \(\PageIndex{2}\)
Saturation/Unsaturation
A saturated fatty acid is one that contains the maximum number of hydrogens possible, and no carbon-carbon double bonds. Carbon normally has four bonds to it. Thus, a saturated fatty acid has hydrogens at every position except carbon-carbon single bonds and carbon-oxygen bonds on the acid end. Two examples of the same 18 carbon saturated fatty acid (stearic acid/stearate) are shown in Figures \(\PageIndex{1}\) and \(\PageIndex{3}\). Figure \(\PageIndex{3}\) is the simplified view of this fatty acid.

Unsaturation means the fatty acid doesn't contain the maximum number of hydrogens on each of its carbons. Instead, unsaturated fatty acids contain a carbon-carbon double bond and only 1 hydrogen off each carbon. The simplest example of unsaturation is a monounsaturated fatty acid. Mono means one, so these are fatty acids with one degree of unsaturation, or one double bond (shown below).
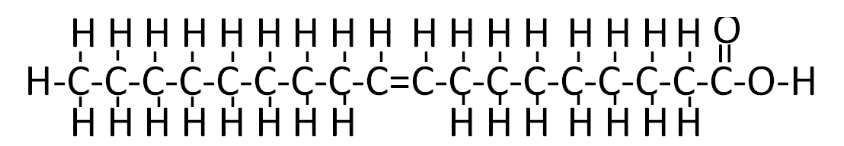
Any fatty acid that has two or more double bonds is considered a polyunsaturated fatty acid. As you may remember from the polysaccharide section, poly means many. A simple example of a polyunsaturated fatty acid is linoleic acid (shown below).

Query \(\PageIndex{3}\)
Double Bond Configuration (Shape)
Double bonds in unsaturated fatty acids are in one of two structural orientations: cis or trans. In a trans orientation, the hydrogens on the carbons involved in the double bond are opposite of one another. In the cis orientation the hydrogens are on the same side of the bond. Steric hindrance in the cis orientation causes the chain to take on a more bent shape.
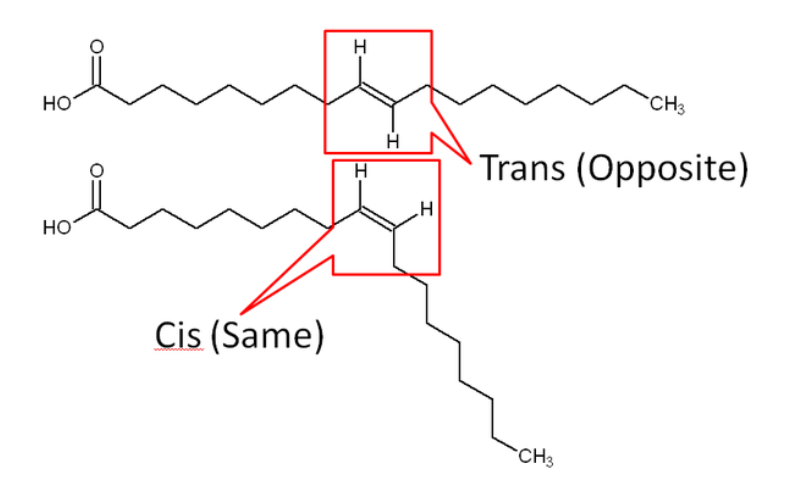
Most natural unsaturated fatty acids are in the cis conformation. As can be seen in Figure \(\PageIndex{7}\), the cis fatty acids have a more of kinked shape, which means they do not pack together as well as the saturated or trans fatty acids. As a result, the melting point is much lower for cis fatty acids compared to trans and saturated fatty acids. To illustrate this difference, the figure below shows the difference in the melting points of saturated, trans-, and cis-monounsaturated 18 carbon fatty acids.
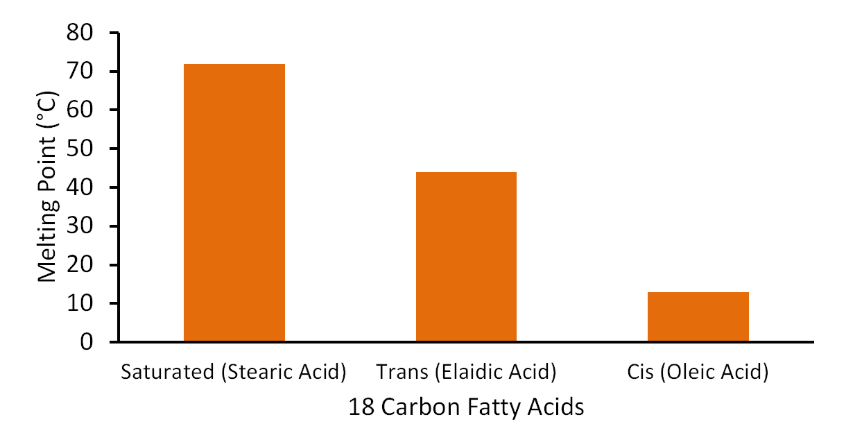
Query \(\PageIndex{4}\)
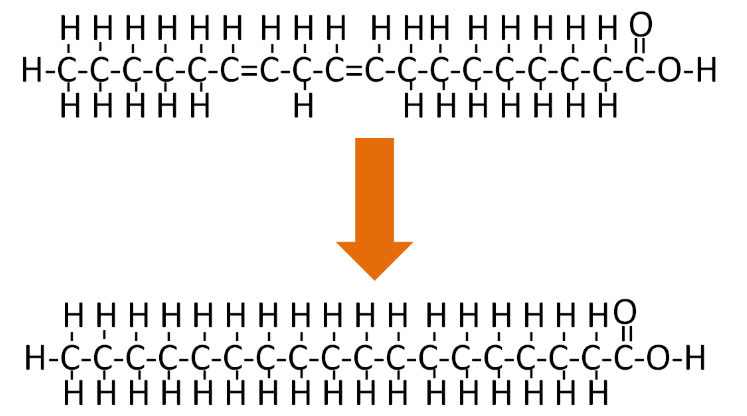
There are some naturally occurring trans fatty acids, such as conjugated linoleic acid (CLA), in dairy products. However, for the most part, trans fatty acids in our diets are not natural; instead, they have been produced synthetically. The primary source of trans fatty acids in our food supply is partially hydrogenated vegetable oil. The 'hydrogenated' means that the oil has gone through the process of hydrogenation. Hydrogenation, like the name implies, is the addition of hydrogen. If an unsaturated fatty acid is completely hydrogenated it would be converted to a saturated fatty acid as shown below.
However, this isn't/wasn't always desirable, thus partially hydrogenated vegetable oil became widely used. To visualize the difference in the amount of hydrogenation consider the difference between tub margarine and stick margarine.
Stick margarine is more fully hydrogenated leading it to have a much harder texture. This is one of the two reasons to hydrogenate, to get a more solid texture. The second reason is that it makes it more shelf-stable, because the double bond(s) of unsaturated fatty acids are susceptible to oxidation, which causes them to become rancid.
Partial hydrogenation causes the conversion of cis to trans fatty acids along with the formation of some saturated fatty acids. Originally, it was thought that trans fatty acids would be a better alternative to saturated fat (think margarine vs. butter). However, it turns out that trans fat is actually worse than saturated fat in altering biomarkers associated with cardiovascular disease. Trans fat increases low-density lipoprotein and decreases high-density liporotein levels, while saturated fat increases low-density lipoproteins without altering high-density lipoprotein levels. But this does not mean that butter is a better choice than margarine as described in the first link. The FDA revoked Generally Recognized as Safe (GRAS) status of partially hydrogenated vegetable oil as described in the second link, and is requiring its use to be phased out by 2018. After that point, permission will need to be requested to use them in foods.
Query \(\PageIndex{5}\)
Query \(\PageIndex{6A}\)
Query \(\PageIndex{6B}\)
Fatty Acid Naming & Food Sources
There are three naming systems used for fatty acids:
- Delta nomenclature
- Omega nomenclature
- Common names
The omega nomenclature and common names are used more in the field of nutrition than the delta nomenclature when describing specific fatty acids.
Delta Nomenclature
For delta nomenclature you need to know 3 things:
- Number of carbons in the fatty acid
- Number of double bonds
- Number of carbons from the carboxylic acid (alpha) end to the first carbon in the double bond(s)
Let's consider the example in the figure below.
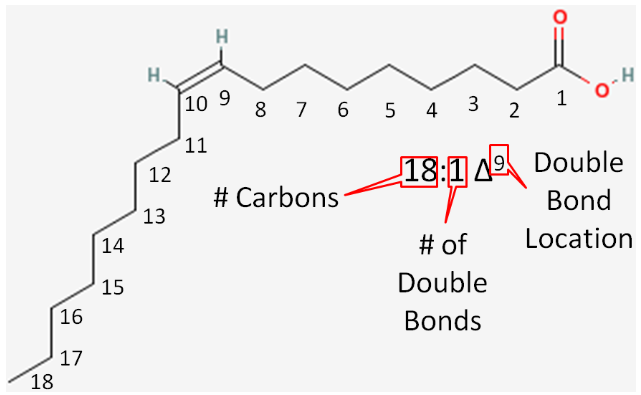
- Number of carbons in the fatty acid = 18
- Number of double bonds = 1
- Number of carbons from the carboxylic acid end to the first carbon in the double bond = 9
This is then written as shown in Figure \(\PageIndex{10}\).
Query \(\PageIndex{7}\)
Omega Nomenclature
The omega nomenclature is almost exactly the same as the delta nomenclature, the only differences being:
- Carbons are counted from the methyl (omega) end instead of the carboxylic acid end
- The omega symbol is used instead of the delta symbol
For omega nomenclature you need to know 3 things:
- Number of carbons in the fatty acid
- Number of double bonds
- Number of carbons from the methyl end (aka Omega end) to the first carbon in the double bond closest to the methyl end
We will again consider the same fatty acid.
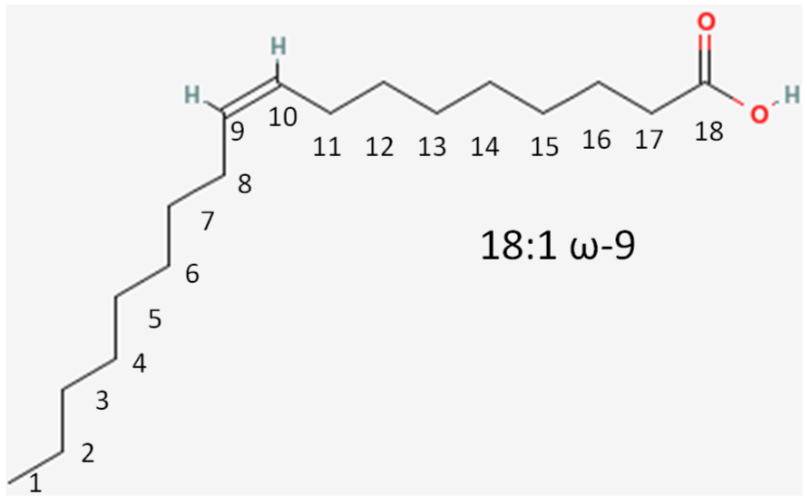
- Number of carbons in the fatty acid = 18
- Number of double bonds = 1
- Number of carbons from the methyl (aka omega) end to the first carbon in the double bond closest to the methyl end = 9
If it is a saturated fatty acid, then the omega nomenclature is not added to the end of the name. If it is an 18 carbon saturated fatty acid, then it would be named 18:0.
This is written as shown in Figure \(\PageIndex{11}\). Instead of an omega prefix, the prefix n- (i.e. n-3) is also commonly used.
Query \(\PageIndex{8}\)
Common Names
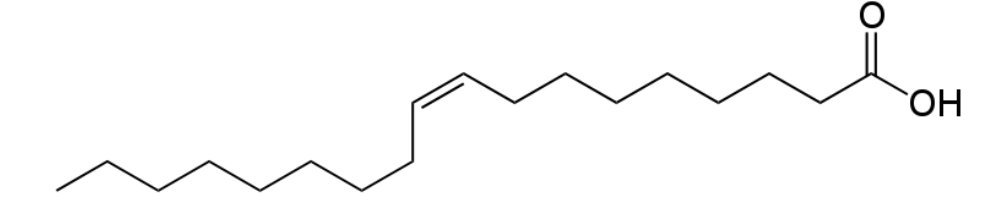
The common names of fatty acids are something that, for the most part, have to be learned/memorized. The common name of the fatty acid we have been naming in this section is oleic acid.
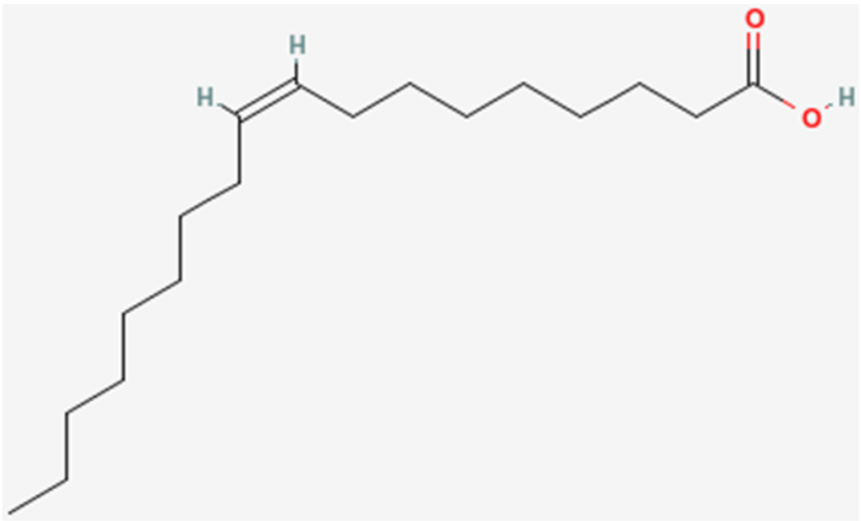
However, it can also be called oleate. The only difference is that, instead of a carboxylic acid on the end of the fatty acid, it has been ionized to form a salt (shown below). This is what the -ate ending indicates and the two names are used interchangeably.
The table below gives the common names and food sources of some common fatty acids.
| Omega Name | Common Name |
|---|---|
| 4:0 | Butyric Acid |
| 12:0 | Lauric Acid |
| 14:0 | Myristic Acid |
| 16:0 | Palmitic acid |
| 18:0 | Stearic Acid |
| 20:0 | Arachidic Acid |
| 24:0 | Lignoceric Acid |
| 18:1 (n-9) | Oleic Acid |
| 18:2 (n-6) | Linoleic Acid |
| 18:3 (n-3) | Alpha-linolenic Acid |
| 20:4 (n-6) | Arachidonic Acid |
| 20:5 (n-3) | Eicosapentanoic Acid |
| 22:6 (n-3) | Docosahexanoic Acid |
The NutritionData link below can help you identify foods that are high in a specific fatty acid.
Web Link
Query \(\PageIndex{9}\)
Food Sources of Fatty Acids
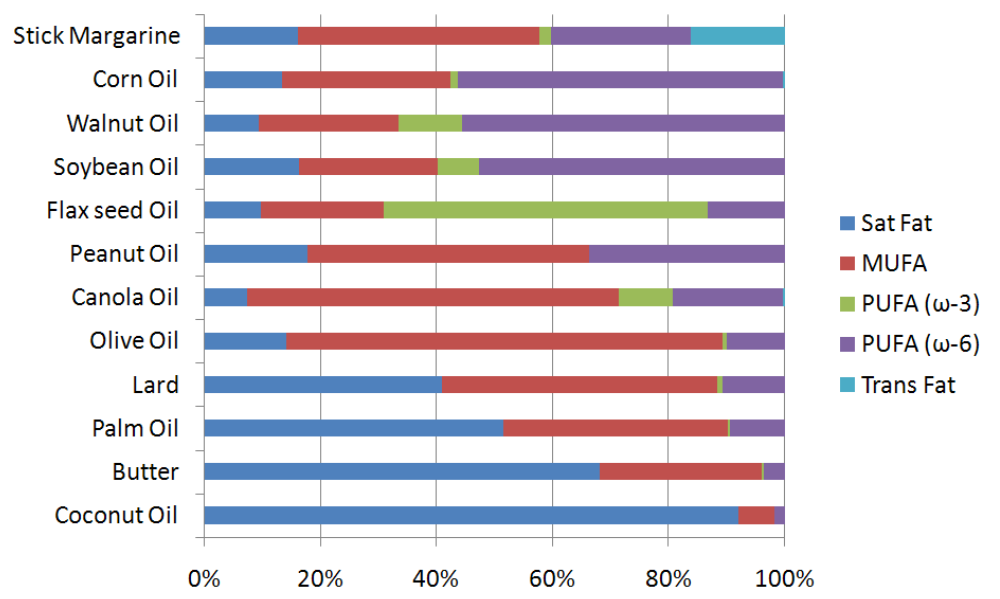
After going through this wide array of fatty acids, you may be wondering where they are found in nature. The figure below shows the fatty acid composition of certain oils and oil-based foods. As you can see, most foods contain a mixture of fatty acids. Stick margarine is the only product in the figure that contains an appreciable amount of trans fatty acids. Corn, walnut, and soybean are rich sources of n-6 polyunsaturated fatty acids, while flax seed is fairly unique among plants in that it is a good source of n-3 polyunsaturated fatty acids. Canola and olive oil are rich sources of monounsaturated fatty acids. Lard, palm oil, butter and coconut oil all contain a significant amount of saturated fatty acids.
Query \(\PageIndex{10}\)
Essential Fatty Acids & Eicosanoids
The two essential fatty acids are:
- linoleic acid (omega-6)
- alpha-linolenic (omega-3)
These fatty acids are essential because we can not synthesize them. This is because we do not have an enzyme capable of adding a double bond (desaturating) at the omega-6 and 3 positions. We do have an enzyme that can add the omega-9 bond, which is why it is not essential. The structures of the two essential fatty acids are shown below.
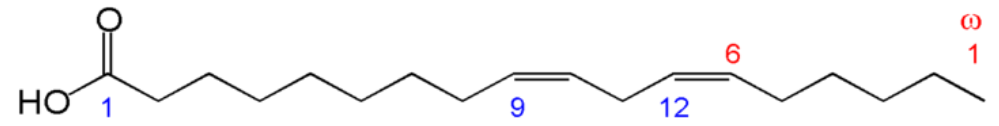
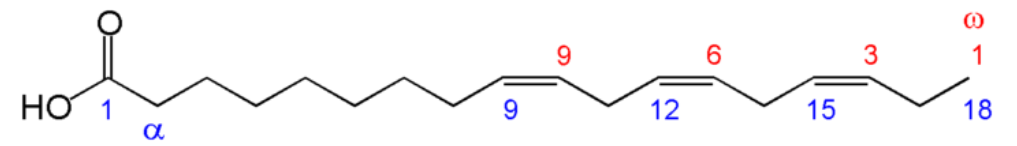
However, we do possess enzymes that can take the essential fatty acids, elongate them (add two carbons to them), and then further desaturate them (add double bonds) to other omega-6 and omega-3 fatty acids. Thus, there are 2 families of fatty acids that the majority of polyunsaturated fatty acids fit into as shown below.

Query \(\PageIndex{11}\)
The same enzymes are used for both omega-6 and omega-3 fatty acids. However, we cannot convert omega-3 fatty acids to omega-6 fatty acids or omega-6 fatty acids to omega-3 fatty acids. Among these families, the omega-3 fatty acid, eicosapentaenoic acid, and the omega-6 fatty acids, dihomo gamma-linolenic acid and arachidonic acid, are used to form compounds known as eicosanoids. These 20 carbon fatty acid derivatives are biologically active in the body (like hormones, but they act locally in the tissue they are produced). There are four classes of eicosanoids:
- Prostaglandins (PG)
- Prostacyclins (PC)
- Thromboxanes (TX)
- Leukotrienes (LT)
Some examples of eicosanoid structures are shown in the figure below:

Query \(\PageIndex{12}\)
The difference in the effects and outcomes of omega-6 and omega-3 fatty acid intake is primarily a result of the eicosanoids produced from them. Omega-6 fatty acid derived eicosanoids are more inflammatory than omega-3 fatty acid derived eicosanoids. As a result, omega-3 fatty acids are considered anti-inflammatory because replacing the more inflammatory omega-6 fatty acid derived eicosanoids with omega-3 fatty acid derived eicosanoids will decrease inflammation. As an example of the action of eicosanoids, aspirin works by inhibiting the enzymes cyclooxygenase 1 (Cox-1) and cyclooxygenase 2 (Cox-2). These enzymes convert arachidonic acid into inflammatory prostaglandins as shown below.
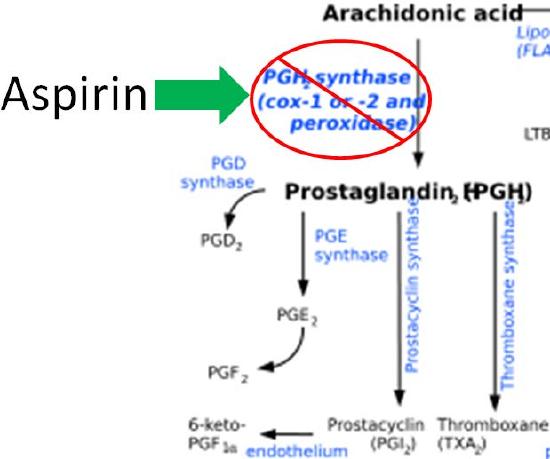
Query \(\PageIndex{13}\)
You have probably heard that you should get more omega-3s in your diet, and in general polyunsaturated fatty acids are considered healthy. However, since omega-3 fatty acids are competing for the same enzymes as omega-6 fatty acids, and because the omega-6 fatty acids are more inflammatory, consuming too many omega-6s is probably more detrimental than helpful. As a result, there is interest in the dietary omega-3:omega-6 fatty acid ratio. For most Americans, the ratio is believed to be too high, at almost 10-20 times more omega-6 fatty acids than omega-3 fatty acids16. The table below shows good food sources of some selected omega-3 and omega-6 fatty acids.
| Fatty Acid | Good Food Sources |
|---|---|
| Linoleic Acid (LA, n-6) | Safflower Oil, Corn Oil, Sunflower Oil |
| Arachidonic Acid (AA, n-6) | Eggs, Meat |
| Alpha-Linolenic Acid (ALA, n-3) | Walnuts, Flaxseed (linseed), Canola (rapeseed), and Soybean Oils |
| Eicosapentaenoic Acid (EPA, n-3) | Fatty Fish & Fish Oils |
| Docosahexanoic Acid (DHA, n-3) | Fatty Fish & Fish Oils |
Even though Figure \(\PageIndex{17}\) illustrates the conversion of alpha-linolenic acid to eicosapentanoic acid and docosahexanoic acid, this conversion is actually quite limited. Only 0.2-8% and 0-4% of alpha-linolenic acid is converted to eicosapentanoic acid and docosahexanoic acid, respectively17. Thus, dietary consumption is the most effective way to get the longer chain fatty acids (eicosapentanoic acid and docosahexanoic acid) in our bodies. It is less clear whether alpha-linolenic acid consumption is as beneficial as eicosapentanoic acid and docosahexanoic acid) but a study found it to be equally effective in decreasing blood triglyceride concentrations. In that study, DHA had the added positive benefit of increasing high-density lipoprotein18. These are all positive outcomes that are expected to reduce the risk of developing cardiovascular disease. However, there is evidence accumulating that there is not much cardiovascular benefit from taking fish oil supplements as described in the article below.
Web Link
Fish Oils Claims Not Supported By Research
Essential Fatty Acid Deficiency
Essential fatty acid deficiency is rare and unlikely to occur, but the symptoms are:
- Growth inhibition
- Reproductive problems
- Skin lesions
- Neurological and visual problems
Query \(\PageIndex{14}\)
Triglycerides
Triglycerides are the most common lipid in our bodies and in the foods we consume. Fatty acids are not typically found free in nature, instead they are found in triglycerides. Breaking down the name triglyceride tells a lot about their structure. "Tri" refers to the three fatty acids, "glyceride" refers to the glycerol backbone that the three fatty acids are bonded to. Thus, a monoglyceride contains one fatty acid, a diglyceride contains two fatty acids. Triglycerides perform the following functions in our bodies:
- Provide energy
- Primary form of energy storage in the body
- Insulate and protect
- Aid in the absorption and transport of fat-soluble vitamins.
A triglyceride is formed by three fatty acids being bonded to glycerol as shown below.
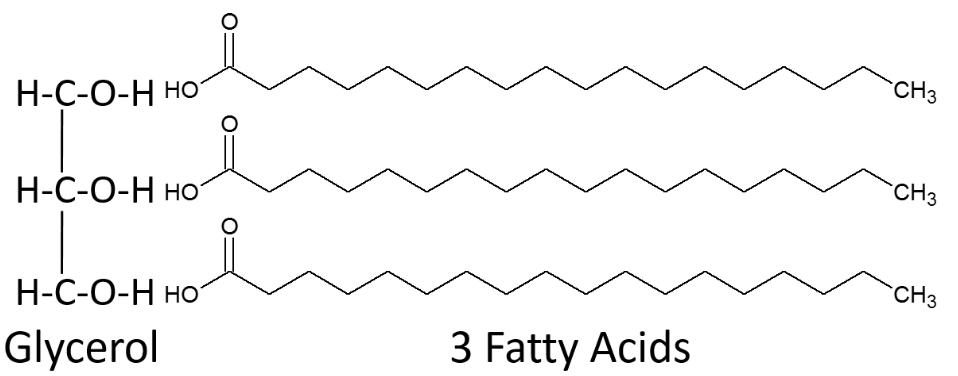
When a fatty acid is added to the glycerol backbone, this process is called esterification. This process is so named because it forms an ester bond between each fatty acid and glycerol. Three molecules of water are also formed during this process as shown below.
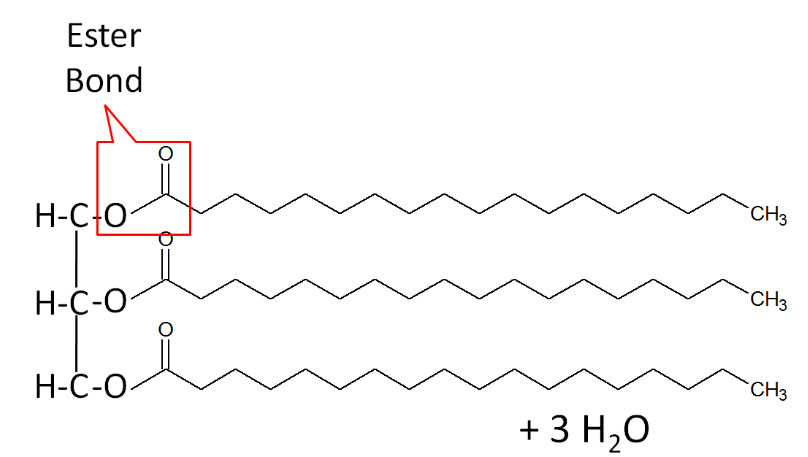
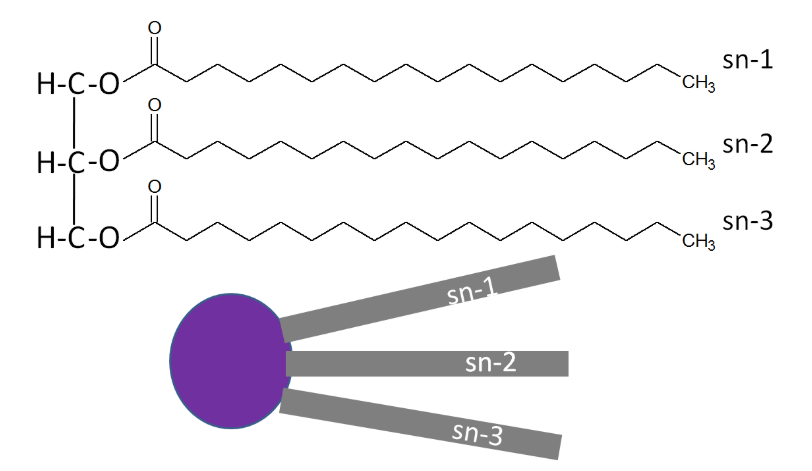
A stereospecific numbering (sn) system is used to number the three fatty acids in a triglyceride sn-1, sn-2, and sn-3 respectively. A triglyceride can also be simply represented as a polar (hydrophilic) head, with 3 nonpolar (hydrophobic) tails, as shown below.
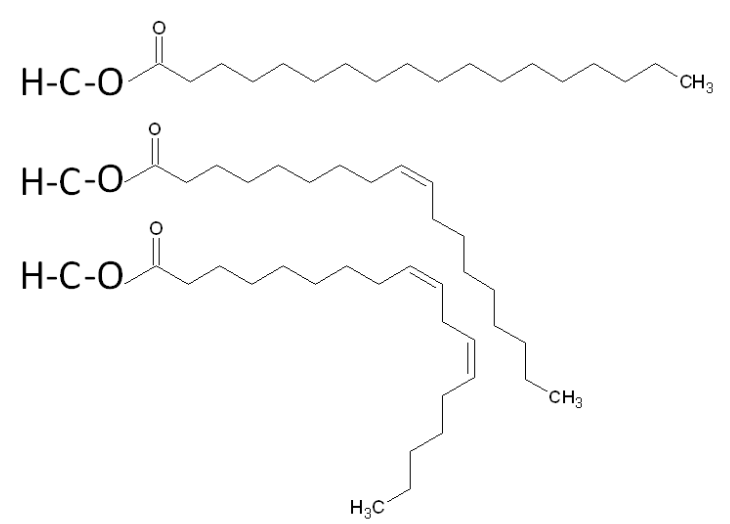
The three fatty acids in a triglyceride can be the same or can each be a different fatty acid. A triglyceride containing different fatty acids is known as a mixed triglyceride. An example of a mixed triglyceride is shown below.
Query \(\PageIndex{15}\)
Phospholipids
Phospholipids are similar in structure to triglycerides, with the only difference being a phosphate group and a nitrogen-containing compound in the place of a fatty acid.
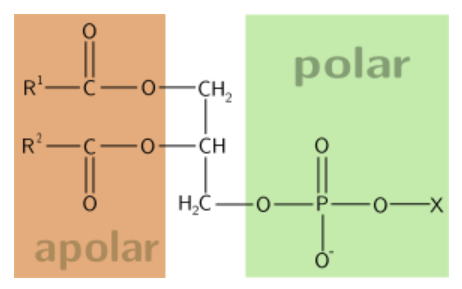
The best known phospholipid is phosphatidylcholine (aka lecithin). As you can see in the structure below, it contains a choline off of the phosphate group.

However, you will not normally find phospholipids arranged like a triglyceride, with the 3 tails opposite of the glycerol head. This is because the phosphate/nitrogen group of the phospholipid is polar. Thus, the structure will look like the 2 figures below.

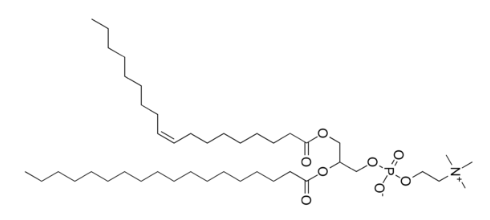
Similar to triglycerides, phospholipids are also represented as a hydrophilic head with two hydrophobic tails as shown below.

Query \(\PageIndex{16}\)
Phospholipid Functions
Because its structure allows it to be at the interface of water-lipid environments, there are two main functions of phospholipids:
- Key Component of Cells' Lipid Bilayers
- Emulsification
Number 1 in the figure below is a cell's lipid bilayer, while 2 is a micelle that is formed by phospholipids to assist in emulsification.
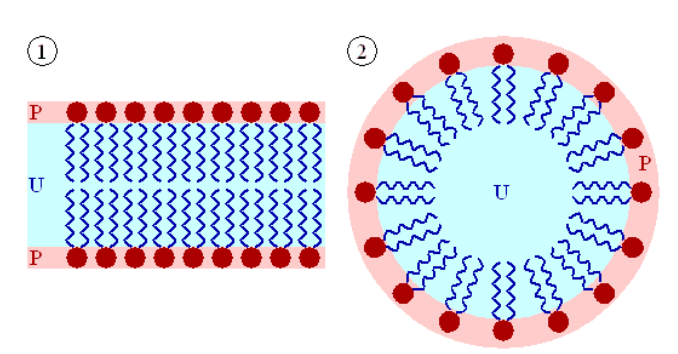
1. Key Component of Cells' Lipid Bilayers
Phospholipids are an important component of the lipid bilayers of cells. A cross section of a lipid bilayer is shown below. The hydrophilic heads are on the outside and inside of the cell; the hydrophobic tails are in the interior of the cell membrane.

2. Emulsification
As emulsifiers, phospholipids help hydrophobic substances mix in a watery environment because of their amphipathic (has hydrophobic and hydrophilic) properties. It does this by forming a micelle as shown below. The hydrophobic (water fearing) substance is trapped on the interior of the micelle away from the aqueous environment.

As a result, it can take a hydrophobic liquid (oil) and allow it to mix with hydrophilic (water loving) liquid (water).

Foods rich in phosphatidylcholine include: egg yolks, liver, soybeans, wheat germ, and peanuts8. Egg yolks serve as an emulsifier in a variety of recipes. Your body makes all the phospholipids that it needs, so they do not need to be consumed (not essential).
Query \(\PageIndex{17}\)
Sterols
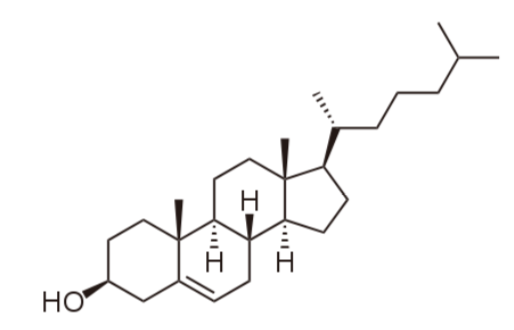
The last category of lipids are the sterols. Their structure is quite different from the other lipids because sterols are made up of a number of carbon rings. The generic structure of a sterol is shown below.

The primary sterol that we consume is cholesterol. The structure of cholesterol is shown below.
Cholesterol is frequently found in foods as a cholesterol ester, meaning that there is a fatty acid attached to it. The structure of a cholesterol ester is shown below.
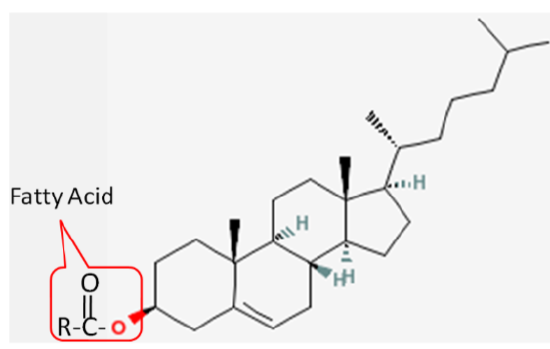
All sterols have a similar structure to cholesterol. Cholesterol is only found in foods of animal origin. If consumers were more knowledgeable, intentionally misleading practices, such as labeling a banana “cholesterol free”, would not be as widespread as they currently are today.
Function
Although cholesterol has acquired the status of a nutrition "villain", it is a vital component of cell membranes and is used to produce vitamin D, hormones, and bile acids. You can see the similarity between the structures of vitamin D and estradiol, one of the forms of estrogen shown below.

We do not need to consume any cholesterol from our diets (not essential) because our bodies have the ability to synthesize the required amounts. The figure below gives you an idea of the cholesterol content of a variety of foods.
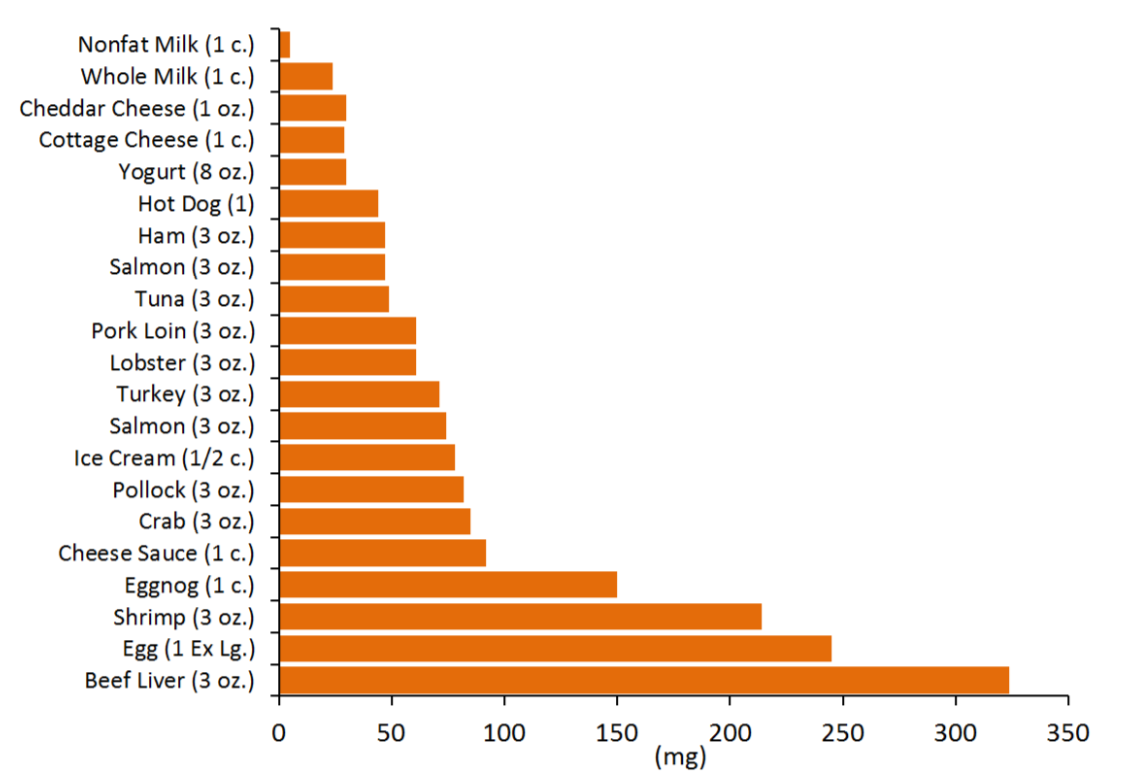
There is neither bad nor good cholesterol, despite these descriptions being commonly used for low-density lipoprotein and high-density lipoprotein, respectively. Cholesterol is cholesterol. High-density lipoprotein and low-density lipoprotein contain cholesterol but are actually lipoproteins that are described in chapter 4.
Query \(\PageIndex{18}\)
References
- Beare-Rogers J, Dieffenbacher A, Holm JV. (2001) Lexicon of lipid nutrition. Pure Appl Chem 73(4): 685-744.
- en.Wikipedia.org/wiki/File:Stearic_acid.svg
- en.Wikipedia.org/wiki/Oleic_...D-skeletal.png
- en.Wikipedia.org/wiki/File:Linoleic_acid.png
- Gropper SS, Smith JL, Groff JL. (2008) Advanced nutrition and human metabolism. Belmont, CA: Wadsworth Publishing.
- www.nutritiondata.com
- en.Wikipedia.org/wiki/File:LAnumbering.png
- en.Wikipedia.org/wiki/File:ALAnumbering.png
- en.Wikipedia.org/wiki/File:EF...icosanoids.svg
- en.Wikipedia.org/wiki/File:Pr...glandin_E1.svg
- en.Wikipedia.org/wiki/File:Thromboxane_A2.png
- en.Wikipedia.org/wiki/File:Leukotriene_B4.svg
- en.Wikipedia.org/wiki/File:Pr...glandin_I2.png
- en.Wikipedia.org/wiki/File:Leukotriene_E4.svg
- en.Wikipedia.org/wiki/File:Ei..._synthesis.svg
- Simopoulos AP. (2008) The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med 233(6): 674.
- Arterburn LM, Hall EB, Oken, H. (2006) Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr 83(suppl) 1467.
- Egert S, Kannenberg F, Somoza V, Erbersdobler H, Wahrburg U. (2009) Dietary alpha-linolenic acid, EPA, and DHA have differential effects on LDL fatty acid composition but similar effects on serum lipid profiles in normolipidemic humans. J Nutr 139(5): 861.
- en.Wikipedia.org/wiki/File:Phospholipid.svg
- commons.wikimedia.org/wiki/Fi...pc_details.svg
- en.Wikipedia.org/wiki/File:Ph...dylcholine.png
- en.Wikipedia.org/wiki/File:Li...nd_micelle.png
- en.Wikipedia.org/wiki/File:Bi...on_profile.svg
- en.Wikipedia.org/wiki/Micell..._scheme-en.svg
- en.Wikipedia.org/wiki/File:Emulsions.svg
- Byrd-Bredbenner C, Moe G, Beshgetoor D, Berning J. (2009) Wardlaw's perspectives in nutrition. New York, NY: McGraw-Hill.
- en.Wikipedia.org/wiki/File:Cholesterol.svg
- en.Wikipedia.org/wiki/File:Cholecalciferol.svg
- en.Wikipedia.org/wiki/File:Estradiol2.png
- http://ndb.nal.usda.gov/


