10.5: Niacin
- Page ID
- 40984
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)There are two forms of niacin: nicotinic acid and nicotinamide (aka niacinamide), that have a carboxylic acid group or amide group, respectively. The structure of nicotinic acid and nicotinamide are shown below.
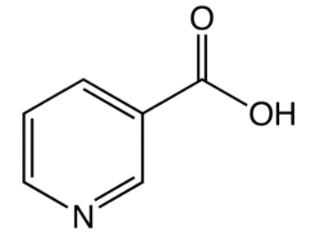
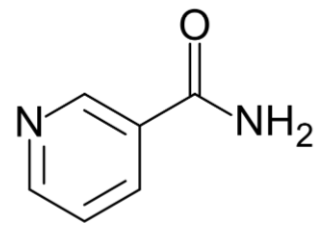
Query \(\PageIndex{1}\)
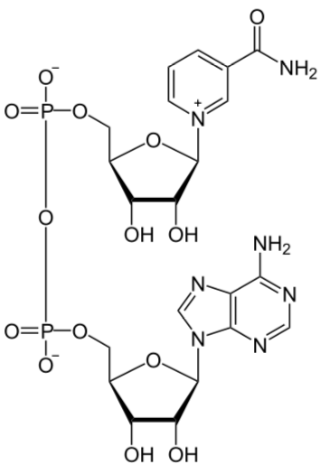
Niacin is important for the production of two cofactors: nicotinamide adenine dinucleotide (\(\ce{NAD}\)) and nicotinamide adenine dinucleotide phosphate (\(\ce{NADP+}\)). The structure of \(\ce{NAD}\) is shown below; you can clearly see the nicotinamide at the top right of the molecule.
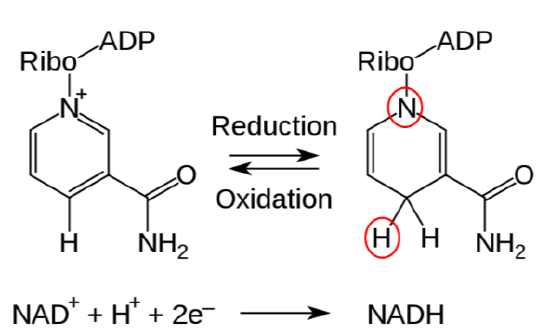
\(\ce{NAD}\) is reduced to form \(\ce{NADH}\), as shown below.
The structure of \(\ce{NADP+}\) is exactly the same as \(\ce{NAD}\), except it has an extra phosphate group off the bottom of the structure, as shown below.
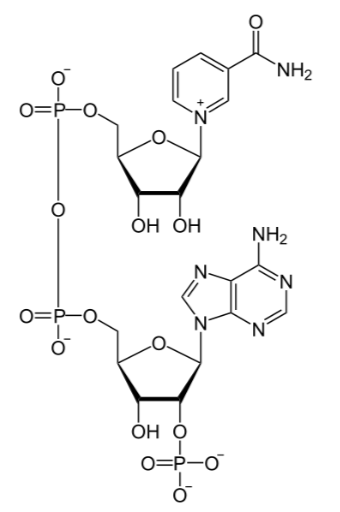
Like \(\ce{NAD}\), \(\ce{NADP+}\) can be reduced to \(\ce{NADPH}\).
Query \(\PageIndex{2}\)
Niacin is unique in that it can be synthesized from the amino acid tryptophan as shown below. An intermediate in this synthesis is kynurenine. Many reactions occur between this compound and niacin, and riboflavin and vitamin \(B_6\) are required for two of these reactions.
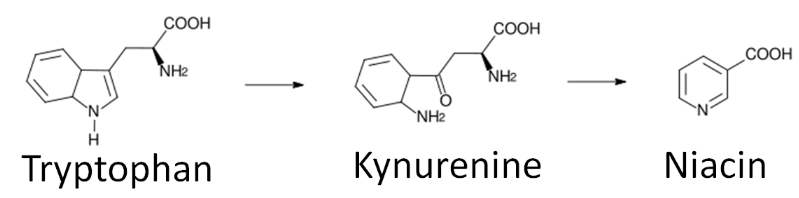
To account for niacin synthesis from tryptophan, niacin equivalents (NE) were created by the DRI committee to account for the amount of niacin in foods as well as their tryptophan content. It takes approximately 60 mg of tryptophan to make 1 mg of niacin. Thus, the conversions to niacin equivalents are:
\[1\, mg\, \text{Niacin} = 1\, NE\]
\[60\, mg\, \text{Tryptophan} = 1\, NE \label{eq2}\]
The tryptophan levels of most foods is not known, but a good estimate is that tryptophan is 1% of amino acids in protein7. Thus, lets take peanut butter, smooth style, with salt as an example8.
Example \(\PageIndex{1}\): Peanut Butter
The peanut butter contains 13.403 mg of niacin and 25.09 g of protein8.
Step 1: Calculate the amount of tryptophan:
\[25.09\, g \times 0.01 \text{(the numerical value of 1%)} = 0.2509\,g \, \text{of tryptophan} \nonumber\]
Step 2: Convert Grams to Milligrams
\[0.2509\, g \times 1000\, mg/g = 250.9\, mg\, \text{of tryptophan} \nonumber\]
Step 3: Calculate NE from tryptophan (Equation \ref{eq2})
\[\dfrac{250.9\, mg \,\text{of tryptophan}}{60\, mg\, \text{of tryptophan}/1 NE} = 4.182 \,NE \nonumber\]
Step 4: Add NEs together
\[13.403\, NE\, \text{(from niacin)} + 4.182 \, \text{(from tryptophan)} = 17.585\, NE \nonumber\]
Query \(\PageIndex{3}\)
Most niacin we consume is in the form of nicotinamide and nicotinic acid9, and in general is well absorbed using an unresolved carrier10. However, in corn, wheat, and certain other cereal products, niacin bioavailability is low. In these foods, some niacin (~70% in corn) is tightly bound, making it unavailable for absorption. Treating the grains with a base frees the niacin and allows it to be absorbed. After absorption nicotinamide is the primary circulating form7,9.
Query \(\PageIndex{4}\)
Niacin Functions
Approximately 200 enzymes require \(\ce{NAD}\) or \(\ce{NADP+}\). We will go through some selected functions of \(\ce{NAD}\) and \(\ce{NADP+}\). The following figures and legends show and describe the functions of \(\ce{NAD}\) and \(\ce{NADP+}\).


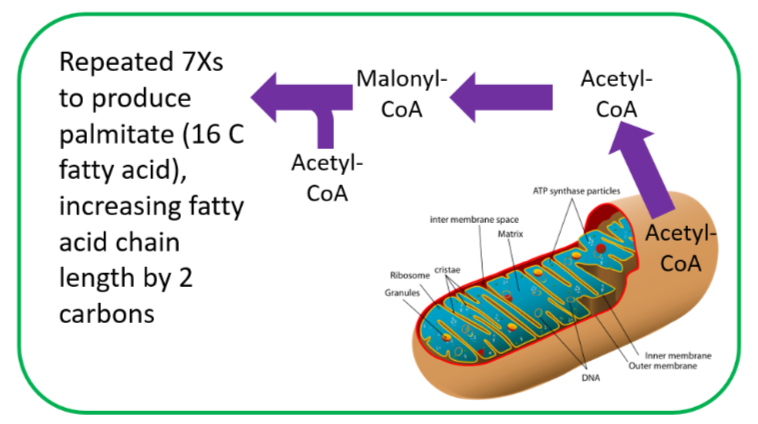
HMG \(\ce{CoA}\) reductase, the rate-limiting enzyme in cholesterol synthesis, uses \(\ce{NADPH}\). \(\ce{NADPH}\) is also used by the antioxidant enzyme glutathione reductase as shown in the link below9.
Query \(\PageIndex{5}\)
Niacin Deficiency & Toxicity
Pellagra is a niacin deficiency. This is no longer a common deficiency in developed countries, but was in the U.S. in the early 1900s. This was because corn was a staple crop, meaning it was what people primarily consumed. The bioavailability of niacin from corn is poor unless treated with a base to release the bound niacin. The symptoms of pellagra are the 3 D's:
- Dementia
- Dermatitis
- Diarrhea
Some refer to 4 D's in which the 4th D is death if the condition is not managed. The following pictures show the symptoms of pellagra.
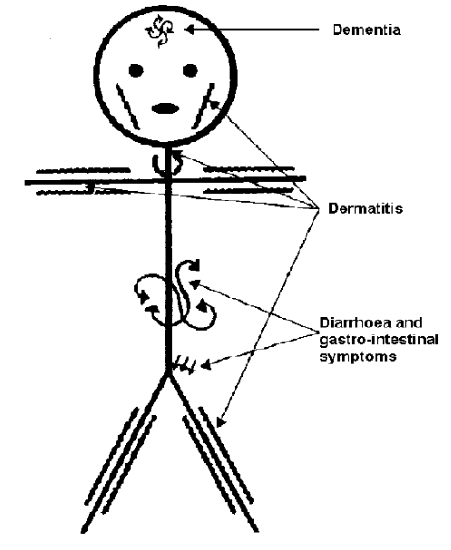

The link below shows another picture of a person suffering from dermatitis as a result of pellagra.
Web Link
Query \(\PageIndex{6}\)
Dietary niacin toxicity is rare. However, nicotinic acid (not) can improve people's lipid profiles when consumed at levels far above the RDA. For instance the RDA and upper limit (UL) is 14 or 16 (women & men) and 35 mg (both), respectively. Many people are taking 1-2 grams (up to 6 g/day) to get the benefits in their plasma lipid profiles as shown in the table below7,9.
| Measure | Change |
|---|---|
| VLDL | ↓ 25-40% |
| LDL | ↓ 6-22% |
| HDL | ↑ 18-35% |
| Total Cholesterol | ↓ 21-44% |
| Triglycerides | ↓ 21-44% |
It should be pointed out that there are special supplements for this purpose that include a slower release nicotinic acid that helps prevent the toxicity symptoms (nicotinamide is not toxic). A slow (aka long or extended) release form of niacin for people with atherosclerosis is Niaspan®. The link below is to Niaspan’s site.
Web Link
A study found that Niaspan plus a statin was no better than a statin alone in preventing heart attacks, despite improvements in HDL and triglyceride concentrations. This result challenged the understanding of the importance of HDL and triglyceride concentrations to heart attack risk. The link below explains this study’s results.
The most well known of the toxicity symptoms is "niacin flush", which is a dilation of capillaries accompanied by tingling that can become painful. This symptom is noted to occur at lower levels than the other toxicity symptoms19. Other symptoms include:
- Gastrointestinal Distress
- Liver Damage
A nicotinic acid receptor GPR109A, which is present in adipocytes and immune cells, is believed to mediate niacin flush, but the beneficial effects on lipid profiles do not appear to be mediated by it20,21. It is not clear at this time the mechanism of action for the improvements in lipid profiles20. However, it appears that the nicotinic acid receptor stated above (GPR109A) increases glucose uptake in the intestine. Pharmacological levels of 1-3g of nicotinic acid administration has been found to result in a rapid reduction of high levels of blood lipids22. However, the increased glucose uptake and rapid reduction in lipid levels has been suggested to lead to a prediabetic state when ingesting these high pharmacological levels more than 100 times the RDA. Thus, nicotinic acid administration needs to be carefully prescribed and monitored by a physician.
Based on the benefits of nicotinic acid supplementation on blood lipid level, you might think this is an ideal candidate for treatment of diabetes or cardiovascular disease. Research investigating nicotinic acid supplementation on both of these disease states has found positive and negative impacts on participants. Although, at first glance nicotinic acid supplementation appears very promising, the detrimental effects on the glucose system are too great for nicotinic acid to be a long term cure. Several clinical trials have investigated the impacts of nicotinic acid supplementation in diabetic patients and patients with cardiovascular disease. The primary outcome of improved lipid levels was observed, however, at the cost of glucose response. Multiple studies observed an increase in the risk of developing diabetes following nicotinic acid supplementation. The lipid levels were corrected, albeit at the expense of poor glucose function. This research has shown the importance of nicotinic acid in improving lipid levels and therefore is currently used in combination with other drugs to improve lipid levels in patients23.
Query \(\PageIndex{7}\)
References
- en.Wikipedia.org/wiki/File:Niacinstr.png
- en.Wikipedia.org/wiki/File:Ni..._structure.svg
- en.Wikipedia.org/wiki/File:NAD%2B_phys.svg
- en.Wikipedia.org/wiki/File:NA..._reduction.svg
- en.Wikipedia.org/wiki/File:NADP%2B_phys.svg
- commons.wikimedia.org/wiki/F...synthesis2.png
- Byrd-Bredbenner C, Moe G, Beshgetoor D, Berning J. (2009) Wardlaw's perspectives in nutrition. New York, NY: McGraw-Hill.
- https://fdc.nal.usda.gov/fdc-app.html#/food-details/784416/nutrients
- Gropper SS, Smith JL, Groff JL. (2008) Advanced nutrition and human metabolism. Belmont, CA: Wadsworth Publishing.
- Said H, Mohammed Z. (2006) Intestinal absorption of water-soluble vitamins: An update. Curr Opin Gastroenterol 22(2): 140-146.
- en.Wikipedia.org/wiki/File:CellRespiration.svg
- en.Wikipedia.org/wiki/File:Ci...conitate_2.svg
- en.Wikipedia.org/wiki/File:Et..._structure.png
- en.Wikipedia.org/wiki/Acetal...de-2D-flat.svg
- en.Wikipedia.org/wiki/Mitochondrion
- https://courses.lumenlearning.com/suny-nutrition/chapter/10-52-niacin-deficiency-toxicity/
- en.Wikipedia.org/wiki/File:Pellagra_NIH.jpg
- Gille A, Bodor E, Ahmed K, Offermanns S. (2008) Nicotinic acid: Pharmacological effects and mechanisms of action. Annu Rev Pharmacol Toxicol 48: 79-106.
- Whitney E, Rolfes SR. (2008) Understanding nutrition. Belmont, CA: Thomson Wadsworth.
- Ginsberg HN, Reyes-Soffer G.. (2013) Niacin: A long history, but a questionable future. Curr Opin Lipidol. 24: 475-479.
- Liu D, Wang X, Kong L, Chen Z. (2015) Nicotinic acid regulates glucose and lipid metabolism through lipid-independent pathways. Curr Pharm Biotechnol. 16: 3-10.
- Meyer-Ficca M, Kirkland JB. (2016). Niacin. Advances in Nutrition, 7:3, 556–558.
- Zeman M, Vecka M, Perlík F, et al. (2015). Niacin in the Treatment of Hyperlipidemias in Light of New Clinical Trials: Has Niacin Lost its Place?. Med Sci Monit. 21:2156-2162.


