12.2: Calcium
- Page ID
- 40994
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Calcium is a macromineral and the most abundant mineral in the body. The reason for calcium’s abundance is its distribution in the skeleton, which contains 99% of the calcium in the body.
Calcium Absorption
Calcium is taken up into the enterocyte through Transient Receptor Potential V6 (TRPV6), a calcium channel found on the brush border. Calbindin is the calcium binding protein that facilitates uptake through TRPV6 and transport across the enterocyte. \(\ce{Ca^2+}-\ce{Mg^2+}\) ATPase functions to pump calcium out of the enterocyte and into circulation and to pump magnesium into the enterocyte, as shown below1.
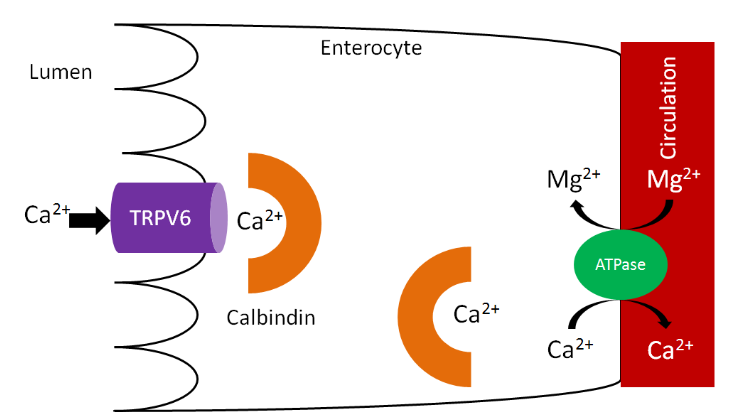
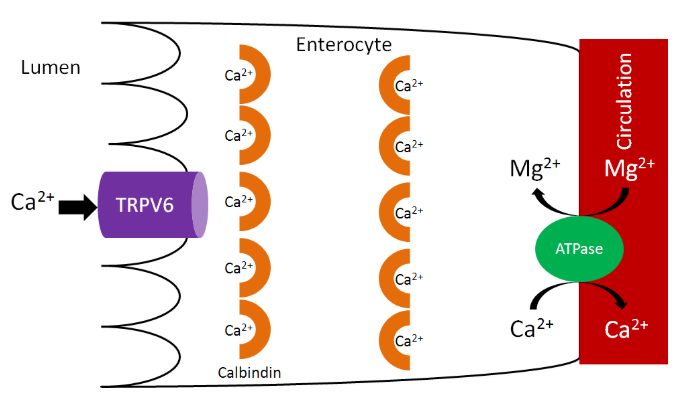
As we have previously discussed, increased 1,25(\(\ce{OH}\))2D synthesis in the kidney causes increased binding to the vitamin D receptor, which increases calbindin synthesis. Increased calbindin ultimately increases calcium uptake and absorption.
Query \(\PageIndex{1}\)
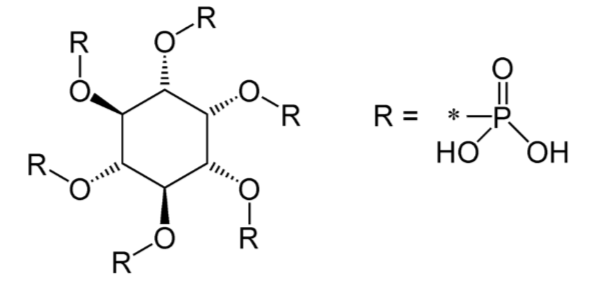
There are a couple of calcium-binding compounds that inhibit its absorption (normally by binding to it). Therefore, even though some foods are good sources of calcium, the calcium is not very bioavailable. Oxalate, found in high levels in spinach, rhubarb, sweet potatoes, and dried beans, is the most potent inhibitor of calcium absorption2. Recall that calcium oxalate is one of the compounds that makes up kidney stones. Based on this understanding, it should not be a surprise that formation of this compound inhibits calcium absorption.

Another inhibitor of calcium absorption is phytate. Phytate is found in whole grains and legumes. You will learn more about it in the phosphorus section.
Query \(\PageIndex{2}\)
Calcium Bioavailability
Calcium bioavailability varies greatly from food to food, as shown in the table below. This table gives the serving size, calcium content of that food, and percent absorbed. The calcium content is multiplied by the absorption percentage to calculate the estimated calcium absorbed. Finally, it shows servings of each food needed to equal the estimated calcium absorbed from 1 serving of milk.
| Food | Serving Size (g) | Calcium content (mg) | Absorption (%) | Estimated Calcium Absorbed | Servings needed to equal 240 mL cow’s milk |
|---|---|---|---|---|---|
| Cow’s Milk | 240 | 300 | 32.1 | 96.3 | 1.0 |
| Almonds, dry roasted | 28 | 80 | 21.2 | 17.0 | 5.7 |
| Beans, Pinto | 86 | 44.7 | 26.7 | 11.9 | 8.1 |
| Beans, Red | 172 | 40.5 | 24.4 | 9.9 | 9.7 |
| Beans, White | 110 | 113 | 21.8 | 24.7 | 3.9 |
| Bok Choy | 85 | 79 | 53.8 | 42.5 | 2.3 |
| Broccoli | 71 | 35 | 61.3 | 21.5 | 4.5 |
| Brussel Sprouts | 78 | 19 | 63.8 | 12.1 | 8.0 |
| Cabbage, Chinese | 85 | 79 | 53.8 | 42.5 | 2.3 |
| Cabbage, Green | 75 | 25 | 64.9 | 16.2 | 5.9 |
| Cauliflower | 62 | 17 | 68.6 | 11.7 | 8.2 |
| Cheddar Cheese | 42 | 303 | 32.1 | 97.2 | 1.0 |
| Chinese mustard greens | 85 | 212 | 40.2 | 85.3 | 1.1 |
| Chinese spinach | 85 | 347 | 8.36 | 29 | 3.3 |
| Fruit Punch (CCM) | 240 | 300 | 52 | 156 | 0.6 |
| Kale | 85 | 61 | 49.3 | 30.1 | 3.2 |
| Kohlrabi | 82 | 20 | 67.0 | 13.4 | 7.2 |
| Mustard Greens | 72 | 64 | 57.8 | 37.0 | 2.6 |
| Orange juice (CCM) | 240 | 300 | 36.3 | 109 | 0.8 |
| Radish | 50 | 14 | 74.4 | 10.4 | 9.2 |
| Rhubarb | 120 | 174 | 8.54 | 10.1 | 9.5 |
| Rutabaga | 85 | 36 | 61.4 | 22.1 | 4.4 |
| Sesame seeds, no hulls | 28 | 37 | 20.8 | 7.7 | 12.2 |
| Soy milk (tricalcium phosphate) | 240 | 300 | 24.0 | 72.0 | 1.3 |
| Soy milk (calcium carbonate) | 240 | 300 | 21.1 | 66.3 | 1.0 |
| Spinach | 85 | 115 | 5.1 | 5.9 | 16.3 |
| Sweet Potatoes | 164 | 44 | 22.2 | 9.8 | 9.8 |
| Tofu with Ca | 126 | 258 | 31.0 | 80.0 | 1.2 |
| Turnip Greens | 72 | 99 | 51.6 | 51.1 | 1.9 |
| Watercress | 17 | 20 | 67.0 | 13.4 | 7.2 |
| Yogurt | 240 | 300 | 32.1 | 96.3 | 1.0 |
Notice that the foods high in oxalate like spinach, rhubarb, sweet potatoes, and dried beans are poorly absorbed. But there are still a number of calcium sources outside of milk.
Query \(\PageIndex{3}\)
The 2 most common forms of calcium found in supplements are calcium carbonate and calcium citrate. As you can see in the figure below, they differ in the amount of elemental calcium they contain. This shows how much of the molecular weight of the compound is calcium.
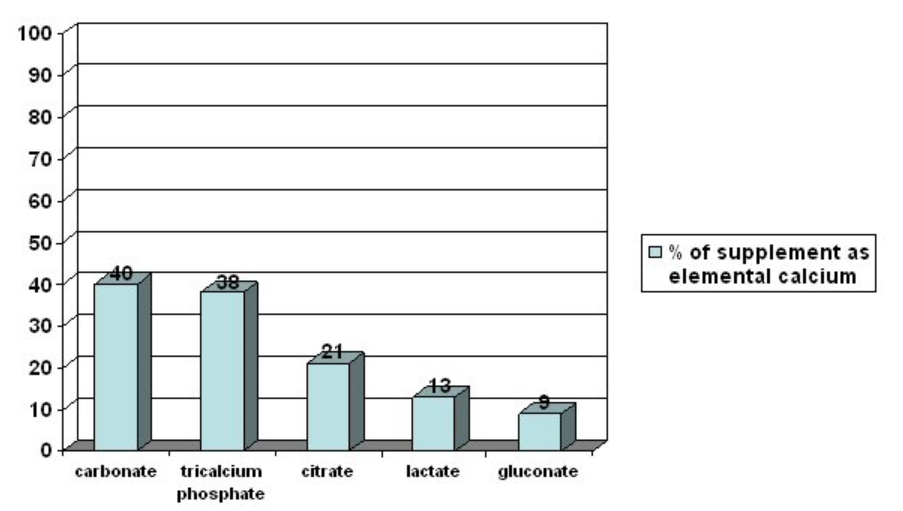
The higher the percent elemental calcium, the greater the amount of calcium you will receive per given weight of that compound, versus a compound that has a lower elemental calcium percentage. Both carbonate and citrate forms are well absorbed, but individuals with low stomach acid absorb citrate better. Also, carbonate is best absorbed when taken with food, while for citrate it is equally well absorbed when taken alone8.
Older research suggested that calcium citrate malate was more bioavailable than other calcium sources. However, a more recent clinical study found no difference in the bioavailability of calcium from calcium citrate malate in orange juice, skim milk, or calcium carbonate supplements9. There is some evidence that suggests that even though bioavailability is the same among these different forms, they might not be equally effective in improving bone measures10.
Query \(\PageIndex{4}\)
Calcium Functions
Calcium in hydroxyapatite is a major component of bones and teeth.
There are also a number of non-bone functions of calcium. Calcium is an intracellular signaling molecule. Because of this, intracellular calcium is tightly controlled, primarily stored within organelles.
Non-bone functions include:
Neurotransmitter release
Neurotransmitter release is stimulated by the opening of voltage-gated \(\ce{Ca^2+}\) channels. This stimulates the synaptic vesicle to fuse with the axon membrane and release the neurotransmitter into the synapse, as shown below11.
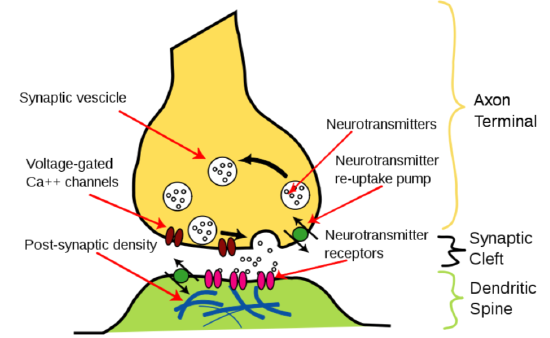
Muscle contraction
Calcium is released in muscle cells, where it binds to the protein troponin, changes its shape, and removes the tropomyosin blockade of actin active sites so that contraction can occur13. This can be seen in the following figure and video.
Hormone release
Calcium acts as an intracellular messenger for the release of hormones, such as insulin. The figure below shows how in the beta cells of the pancreas, the opening of voltage-gated calcium channels stimulates the insulin granules to fuse with the beta cell membrane to release insulin.
Blood Clotting
As will be discussed more in the vitamin K section, calcium binding to activated Gla proteins is important in the blood clotting cascade.
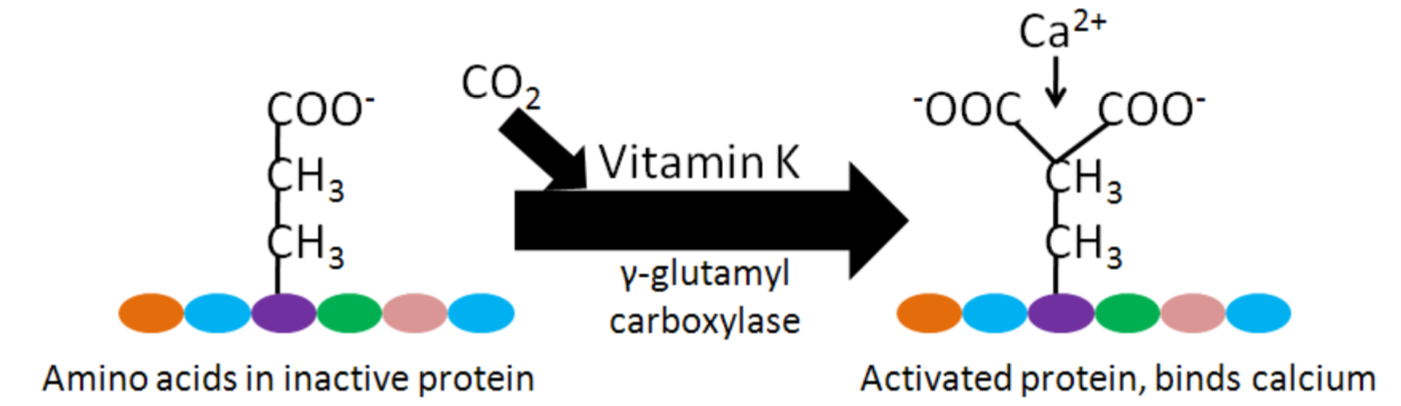
Enzyme regulation
The binding of calcium to calcium-binding proteins also regulates the action of a number of enzymes17.
Query \(\PageIndex{5}\)
Calcium Deficiency & Toxicity
Because of the large amount of calcium in bones (which can be mobilized), deficiency is rare2. Hypocalcemia (low serum blood calcium concentrations) can result in tetany (involuntary muscle contractions)1. In addition, calcium deficiency in children can lead to rickets, the same as occurs in vitamin D deficiency. While not a deficiency, low calcium intake can lead to decreased bone mineral density and the conditions osteopenia and osteoporosis. How these differ from osteomalacia and normal bone is illustrated and described below. There are two different bone components that we will consider to understand what is happening in the bone. Matrix is the protein scaffolding (primarily collagen) onto which mineral is deposited. Mineral is simply the mineral deposited on the matrix.
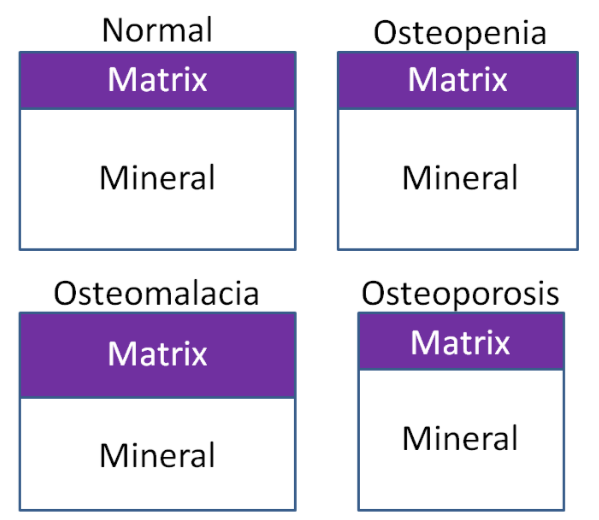
- Osteomalacia - Bone mass is normal, but the matrix to mineral ratio is increased, meaning there is less mineral in bone.
- Osteopenia - Bone mass is decreased, but the matrix to mineral ratio is not altered from normal bone. This condition is intermediate between normal and osteoporosis.
- Osteoporosis - Bone mass is further decreased from osteopenia, but the matrix to mineral ratio is not altered from normal bone16.
Query \(\PageIndex{6}\)
To prevent osteoporosis it is important to build peak bone mass (the maximum amount that a person will have in their lifetime), 90% of which is built by age 18 and 20 in females and males, respectively, but can continue to increase until age 30. After that time, bone mass starts to decrease. For women after menopause, bone mass decreases dramatically because of the decrease in estrogen production, as shown below17.
Query \(\PageIndex{7}\)
There is a decrease after menopause in women (estrogen stimulates osteoblasts), that results in a steep decrease in bone mass. Combined with the fact that women have lower peak bone mass to begin with, helps further explain why osteoporosis is more common in females. A measure of bone status is bone mineral density. As the name indicates, bone mineral density is a measure of the amount of mineral in bone. Dual energy X-ray absorptiometry (DEXA) accurately measures bone mineral density using a small amount of radiation. A DEXA is shown in the figure below.

A person lies down on the table and the arm of the machine moves slowly over them.
From the scan, a bone mineral density t-score is generated. These measure your peak bone density compared to a healthy 30-year old adult and give you values of how much higher or lower you are21. The following table summarizes the levels and their scores.
| Category | T-score |
|---|---|
| Normal | +1 to -1 SD |
| Low bone mass (osteopenia) | -1 to -2.5 |
| Osteoporosis | -2.5 or lower |
This DEXA measurement cannot distinguish whether the low bone mineral density is due to too little bone (osteoporosis) or too little mineral (osteomalacia)21. There are other methods of measuring bone mineral density, such as peripheral DEXA and ultrasound. These typically are done on the wrist or heel, but are not as accurate because that one area might not reflect the bone mineral density in other parts of the body11.
Calcium toxicity is rare, occurring in those with hyperparathyroidism or high calcium supplementation levels. Like vitamin D, toxicity can lead to calcification of soft tissues11. In addition, a very high intake of calcium can lead to kidney stone formation.
Query \(\PageIndex{8}\)
Query \(\PageIndex{9}\)
References
- Gropper SS, Smith JL, Groff JL. (2008) Advanced nutrition and human metabolism. Belmont, CA: Wadsworth Publishing.
- Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, editors. (2006) Modern nutrition in health and disease. Baltimore, MD: Lippincott Williams & Wilkins.
- en.Wikipedia.org/wiki/File:Calcium_oxalate.png
- en.Wikipedia.org/wiki/File:Phytic_acid.png
- Weaver CM, Plawecki KL. (1994) Dietary calcium: Adequacy of a vegetarian diet. Am J Clin Nutr 59(5 Suppl): 1238S-1241S.
- Weaver CM, Proulx WR, Heaney R. (1999) Choices for achieving adequate dietary calcium with a vegetarian diet. Am J Clin Nutr 70(3 Suppl): 543S-548S.
- Weaver C. (2009) Closing the gap between calcium intake and requirements. J Am Diet Assoc 109(5): 812-813.
- http://www.ahs6.com/liquidcalcium/absorb.php
- Martini L, Wood R. (2002) Relative bioavailability of calcium-rich dietary sources in the elderly. Am J Clin Nutr 76(6): 1345-1350.
- Weaver C, Janle E, Martin B, Browne S, Guiden H, et al. (2009) Dairy versus calcium carbonate in promoting peak bone mass and bone maintenance during subsequent calcium deficiency. Journal of Bone and Mineral Research 24(8): 1411-1419.
- Byrd-Bredbenner C, Moe G, Beshgetoor D, Berning J. (2009) Wardlaw's perspectives in nutrition. New York, NY: McGraw-Hill.
- https://courses.lumenlearning.com/suny-nutrition/chapter/12-23-calcium-functions/
- legacy.owensboro.kctcs.edu/GC...ontraction.htm
- https://commons.wikimedia.org/wiki/F...action_new.jpg
- https://commons.wikimedia.org/wiki/F...e_Pancreas.svg
- Sambrook, P. Bone structure and function in normal and disease states v5.books.elsevier.com/booksca...0443070150.pdf
- www.niams.nih.gov/Health_Info.../bone_mass.asp
- https://commons.wikimedia.org/wiki/F..._Bone_Mass.jpg
- en.Wikipedia.org/wiki/File:Bo...ty_scanner.jpg
- www.nof.org/patients/diagnos...y-examtesting/
- https://www.nhs.uk/conditions/dexa-scan/why-its-done/


