Iodine
- Page ID
- 8781
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Iodine is found in the thyroid hormones. Iodine deficiency affects about two billion people and is the leading preventable cause of intellectual disabilities. Iodine is an essential element for life and is the heaviest element commonly needed by living organisms. (Lanthanum and the other lanthanides, as well as tungsten, are used by a few microorganisms.) It is required for the synthesis of the growth-regulating thyroid hormones thyroxine and triiodothyronine (T4 and T3 respectively, named after their number of iodine atoms). A deficiency of iodine leads to decreased production of T3 and T4 and a concomitant enlargement of the thyroid tissue in an attempt to obtain more iodine, causing the disease known as simple goitre. The major form of thyroid hormone in the blood is thyroxine (T4), which has a longer half-life than T3.
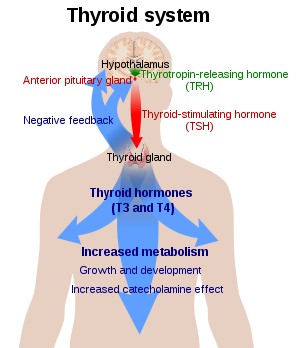
In humans, the ratio of T4 to T3 released into the blood is between 14:1 and 20:1. T4 is converted to the active T3 (three to four times more potent than T4) within cells by deiodinases (5'-iodinase). These are further processed by decarboxylation and deiodination to produce iodothyronamine (T1a) and thyronamine (T0a'). All three isoforms of the deiodinases are selenium-containing enzymes; thus dietary selenium is essential for T3 production.
The thyroid system of the thyroid hormones T3 and T4
Iodine accounts for 65% of the molecular weight of T4 and 59% of T3. Fifteen to 20 mg of iodine is concentrated in thyroid tissue and hormones, but 70% of all iodine in the body is found in other tissues, including mammary glands, eyes, gastric mucosa, fetal thymus, cerebro-spinal fluid and choroid plexus, arterial walls, the cervix, and salivary glands. In the cells of those tissues, iodide enters directly by sodium-iodide symporter (NIS). The action of iodine in mammary tissue is related to fetal and neonatal development, but in the other tissues, it is (at least) partially unknown.
Dietary intake
The daily Dietary Reference Intake recommended by the United States Institute of Medicine is between 110 and 130 µg for infants up to 12 months, 90 µg for children up to eight years, 130 µg for children up to 13 years, 150 µg for adults, 220 µg for pregnant women and 290 µg for lactating mothers.[83] The Tolerable Upper Intake Level (UL) for adults is 1,100 μg/day.[84] The tolerable upper limit was assessed by analyzing the effect of supplementation on thyroid-stimulating hormone.[82] The thyroid gland needs no more than 70 μg/day to synthesise the requisite daily amounts of T4 and T3. The higher recommended daily allowance levels of iodine seem necessary for optimal function of a number of body systems, including lactating breast, gastric mucosa, salivary glands, brain cells, choroid plexus, oral mucosa, and arterial walls.[85][86][87]
Natural sources of dietary iodine include seafood, such as fish, seaweeds (such as kelp) and shellfish, dairy products and eggs so long as the animals received enough iodine, and plants grown on iodine-rich soil.[88][89] Iodised salt is fortified with iodine in the form of sodium iodide.[89][90]
As of 2000, the median intake of iodine from food in the United States was 240 to 300 μg/day for men and 190 to 210 μg/day for women.[84] The general US population has adequate iodine nutrition,[91][92] with women of childbearing age and pregnant women having a possible mild risk of deficiency.[92] In Japan, consumption was considered much higher, ranging between 5,280 μg/day to 13,800 μg/day from dietary seaweed or kombu kelp,[82] often in the form of Kombu Umami extracts for soup stock and potato chips. However, new studies suggest that Japan's consumption is closer to 1,000–3,000 μg/day.[93] The adult UL in Japan was last revised to 3,000 µg/day in 2015.[94]
After iodine fortification programs such as iodisation of salt have been implemented, some cases of iodine-induced hyperthyroidism have been observed (so-called Jod-Basedow phenomenon). The condition seems to occur mainly in people over forty, and the risk appears higher when iodine deficiency is severe and the initial rise in iodine intake is high.[95]
Deficiency
In areas where there is little iodine in the diet,[96] typically remote inland areas and semi-arid equatorial climates where no marine foods are eaten, iodine deficiency gives rise to hypothyroidism, symptoms of which are extreme fatigue, goitre, mental slowing, depression, weight gain, and low basal body temperatures.[97] Iodine deficiency is the leading cause of preventable intellectual disability, a result that occurs primarily when babies or small children are rendered hypothyroidic by a lack of the element. The addition of iodine to table salt has largely eliminated this problem in the wealthier nations, but iodine deficiency remains a serious public health problem today in the developing world.[98] Iodine deficiency is also a problem in certain areas of Europe. Information processing, fine motor skills, and visual problem solving are improved by iodine repletion in moderately iodine-deficient children.[99]
Toxicity
Elemental iodine (I2) is toxic if taken orally undiluted. The lethal dose for an adult human is 30 mg/kg, which is about 2.1–2.4 grams for a human weighing 70 to 80 kg (even if experiments on rats demonstrated that these animals could survive after eating a 14000 mg/kg dose). Excess iodine can be more cytotoxic in the presence of selenium deficiency.[100] Iodine supplementation in selenium-deficient populations is, in theory, problematic, partly for this reason.[82] The toxicity derives from its oxidizing properties, through which it denaturates proteins (including enzymes).[101]
Elemental iodine is also a skin irritant, and direct contact with skin can cause damage and solid iodine crystals should be handled with care. Solutions with high elemental iodine concentration, such as tincture of iodine and Lugol's solution, are capable of causing tissue damage if used in prolonged cleaning or antisepsis; similarly, liquid Povidone-iodine (Betadine) trapped against the skin resulted in chemical burns in some reported cases.[102]
Occupational exposure
People can be exposed to iodine in the workplace by inhalation, ingestion, skin contact, and eye contact. The Occupational Safety and Health Administration (OSHA) has set the legal limit (Permissible exposure limit) for iodine exposure in the workplace at 0.1 ppm (1 mg/m3) during an 8-hour workday. The National Institute for Occupational Safety and Health (NIOSH) has set a Recommended exposure limit (REL) of 0.1 ppm (1 mg/m3) during an 8-hour workday. At levels of 2 ppm, iodine is immediately dangerous to life and health.[103]
Allergic reactions
Some people develop a hypersensitivity to products and foods containing iodine. Applications of tincture of iodine or Betadine can cause rashes, sometimes severe.[104] Parenteral use of iodine-based contrast agents (see above) can cause reactions ranging from a mild rash to fatal anaphylaxis. Such reactions have led to the misconception (widely held, even among physicians) that some people are allergic to iodine itself; even allergies to iodine-rich seafood have been so construed.[105] In fact, there has never been a confirmed report of a true iodine allergy, and an allergy to elemental iodine or simple iodide salts is theoretically impossible. Hypersensitivity reactions to products and foods containing iodine are apparently related to their other molecular components;[106] thus, a person who has demonstrated an allergy to one food or product containing iodine may not be have an allergic reaction to another. Patients with various food allergies (shellfish, egg, milk, etc.) or asthma are more likely to suffer reactions to contrast media containing iodine.[106] As with all medications, the patient's allergy history should be questioned and consulted before any containing iodine are administered.[107]