1.2: Head and Neck Lymph Node and Tumor Biopsy Techniques
- Page ID
- 15427
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)OPEN ACCESS ATLAS OF OTOLARYNGOLOGY, HEAD & NECK OPERATIVE SURGERY
BIOPSY OF HEAD & NECK TUMORS & CERVICAL LYMPH NODES
Johan Fagan, Kathy Taylor, Ellen Bolding
Almost any mass or tumor requires cytological or histological diagnosis before a management plan can be formulated. Performing tissue biopsy of masses and lymph nodes of the head and neck fills many junior doctors with fear due to the complex anatomy and the vascular structures and nerves that traverse the head and neck. Yet diagnostic material can be safely obtained from most masses in the head and neck in an ambulatory care setting.
Techniques and pitfalls to obtain diagnostic material are presented in this chapter. Techniques include brush cytology, fine needle aspiration biopsy/cytology (FNAB/FNAC), core biopsy, punch biopsy, and open surgical biopsy
Tumors of the upper aerodigestive tract are ideally biopsied directly either transorally or transnasally. Subcutaneous tumors are first sampled by the least invasive technique; only if the diagnosis remains in doubt does one employ progressively more invasive biopsy techniques until a diagnosis is made. A typical diagnostic sequence is: FNAC - core biopsy - open surgical biopsy.
What do pathologists require to make a diagnosis?
Clinical detail: A pathologist’s differential diagnosis and types of pathological tests are informed by the clinical information; this is particularly important with FNAC.
FNAC
- Cellular aspirate
- Smear material directly onto slide
- Spray with cytofixative & leave to dry
- Can also rinse needle in 50% alcohol solution or in liquid-based cytology fluid to retrieve cells "stuck" in needle
Tissue biopsy
- Adequate size
- Biopsy technique (not crushed)
- From periphery of specimen (avoids necrotic tumor)
- Place biopsy in 10% formalin for fixation
- If suspect TB or other infective cause, also place a biopsy in sterile saline or culture media for microbiology
Pitfalls and caveats
- Insufficient clinical detail on pathology request form: A pathologist’s differential diagnosis and types of pathological tests can be steered in the right direction by having adequate clinical information; this is particularly important with cytology
- Not excluding a primary tumor prior to proceeding to lymph node biopsy: Especially in non-otolaryngologists’ hands, patients commonly (inappropriately) undergo excision biopsy of a cervical lymph node before having undergone a thorough search for a primary malignancy in the upper aerodigestive tract or skin. Not only may the lymph node biopsy prove to have been unnecessary, but it may also complicate subsequent surgical treatment of the neck
- Not considering infective causes: Diseases such as tuberculosis (TB) and actinomycosis (Figure 1) may masquerade as malignant tumors and lymph node metastases; it is therefore advisable to send a segment of an excised lymph node for TB culture
- Proceeding to open surgical biopsy of a neck mass or lymph node before FNAC: FNAC is cheap, safe and minimally invasive and often yields a diagnosis making open biopsy unnecessary
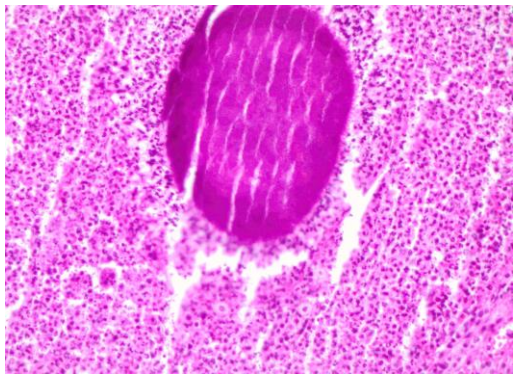
Figure 1: Actinomyces surrounded by neutrophils
- Bloody FNAC aspirate: A bloody smear is often unhelpful; repeat the procedure using a non-aspiration technique
- Inadequate sample size of open tissue biopsy: Having opened the neck, the surgeon should ensure that an adequate volume of tissue is sampled to avoid having to repeat the biopsy
- Taking a biopsy from the necrotic center of a tumor: This applies especially to the oral cavity; biopsy viable cells at the periphery of a tumor
- Non-representative tissue: When a biopsy is being repeated following previous “non-representative” or “inadequate” biopsy, have frozen section done, not to make a diagnosis, but to ensure that pathological tissue has indeed been sampled
- Assuming that a cystic aspirate is infective or benign, and not malignant: Certain malignancies of the head and neck e.g. squamous cell carcinoma (SCC) of the oropharynx and skin, and metastatic melanoma may have cystic lymph node metastases (Figure 2); the appearance of the aspirate may vary from crystal-clear to purulent. Cystic aspirates should therefore always be sent for cytologic examination even when malignancy is not suspected. Smear the aspirate as uniformly as possible (Figure 6). Cystic, well differentiated SCC can also be mistaken for a benign epidermoid or branchial cyst
- Incorrect transport medium: Ensure that tissue sent for histological analysis is placed in formaldehyde in a sealed container; tissue sent for TB culture is transported in normal saline
- Anticoagulation: FNAC can be safely done in patients taking aspirin and nonsteroidal anti-inflammatories. Patients on anticoagulants or with bleeding disorders may present a problem if anticoagulation cannot be safely stopped; ultrasound guided FNAC (USGFNAC) may be appropriate in such cases to avoid puncturing large blood vessels
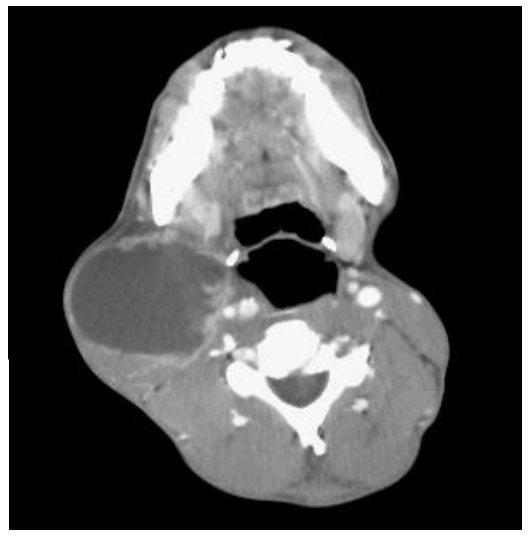
Figure 2: Cystic cervical nodal metastasis originating from squamous cell carcinoma of the tonsil
Fine needle aspiration cytology/biopsy (FNAC/FNAB)
This is a technique whereby a cytologic diagnosis is made on an aspirate of cellular material that has been collected through a small-caliber needle and smeared and fixed on a glass microscope slide. In the absence of an obvious primary tumor, FNAC is generally the 1st line investigation of a neck mass. Because the relationship of tumor cells to the basement membrane cannot be determined on cytology, the cytologist is unable to distinguish between high grade dysplasia (carcinoma in situ) and invasive carcinoma; this distinction can only be made on histological examination of a tissue biopsy that includes the basement membrane.
FNAC is particularly useful in the head and neck for the following reasons:
- Simple, quick, safe and inexpensive
- Requires minimal practice and training
- No anesthesia required (not even local anesthesia)
- Rapid diagnosis
- Insignificant risk of injury to nerves e.g. facial nerve in the parotid gland
- Does not cause seeding of tumor cells
- Insignificant risk of bleeding; if a major vessel e.g. carotid artery or internal jugular vein is punctured with a small gauge needle, bleeding settles with finger pressure applied to the puncture site
- Good yield for metastatic SCC, and for differentiating inflammatory masses from neoplasia
Equipment for FNAC (Figure 3)
- 23-gauge needle: a thin needle causes less bleeding, is less painful, and has a similar diagnostic yield as larger needles
- Syringe (5 or 10 mL)
- Alcohol / iodine gauze swab to sterilize skin
- Gauze swab to compress needle puncture site
- Two glass microscope slides
- Cytology fixative (unless air-dried)
Local anesthesia
The authors do not use local anesthesia as the additional needle stick for the anesthetic also causes discomfort and the anesthetic solution may obscure a small mass.
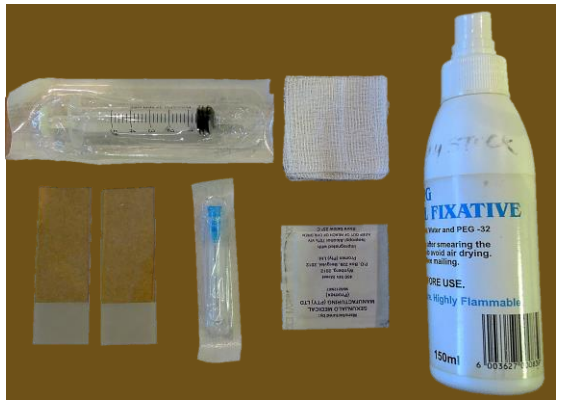
Figure 3: FNAC equipment: syringe, gauze swab, 2 glass microscope slides, 23-gauge needle, alcohol swab, fixative
Accessing the mass
FNAC is done either with the patient sitting in a chair or lying down. With FNAC of the thyroid the neck may be hyperextended over a bolster placed under the shoulders. Access to lymph nodes along the jugular chain may be improved by turning the head. If possible, the mass is fixed between the fingers of the nondominant hand.
Masses in Level 1b of the neck may be more accessible and better stabilized by displacing them inferolaterally with a finger placed in the lateral floor of mouth. Great care should however be taken not to pierce the operator’s finger in the mouth with the tip of the needle.
Ultrasound-guided (USGFNAC) or CT-guided FNAC may be done for masses that are difficult to access with confidence e.g. deep-seated masses in the deep lobe of parotid or parapharyngeal space, or smaller masses.
Harvesting the cellular material
Non-aspiration technique
Also known as the fine-needle capillary method, this is the authors’ preferred method. It relies on capillary action to draw sheared cells up a small-caliber needle. The non-aspiration technique is easier to perform than aspiration technique, improves the operator’s ability to direct the needle tip into a smaller mass, and is less likely to cause a bloody aspirate which is particularly advantageous with vascular structures such as thyroid gland.
- Hold the hub of a 23-gauge needle between index/middle finger and thumb of the non-dominant hand without syringe attached
- Advance the needle through the skin into the mass
- Move the needle back-and-forth with short, rapid strokes while rotating the needle
- Withdraw the needle
- Attach a syringe with plunger retracted, to the hub of the needle
- Carefully eject the material onto a glass microscope slide
Aspiration Technique
The aspiration technique employs negative pressure generated by a syringe as well as the shearing effect of the needle, to aspirate cellular material from a mass. A pistol grip syringe holder can be used as it allows more uniform suction and makes it easier to direct the needle (Figure 4).
- Attach the needle to the syringe
- Insert the needle into the mass without applying suction
- Pull back the plunger of the syringe
- Maintain suction while moving the needle back-and-forth
- Release the plunger of the syringe to relieve the negative pressure
- Withdraw the needle
- Disconnect the needle from the syringe
- Retract the plunger of the syringe to fill it with air and reattach it to the needle
- Eject the aspirated material onto a glass microscope slide

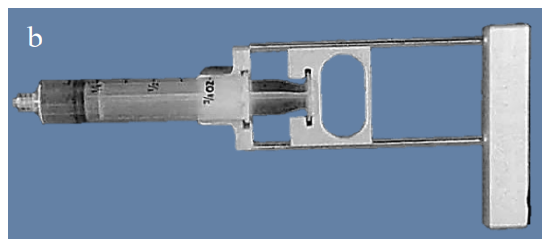
Figures 4a, b: Example of pistol grip syringe holder
Smearing the aspirate
As soon as the aspirate has been ejected onto the glass microscope slide, a 2nd microscope slide is used to smear the cellular material into a monolayer of cells for microscopic examination (Figure 5).
Figure 6 illustrates two techniques that may be used to create a thin film. The “pull-push” smear technique is only applicable to liquid aspirates. The authors favor the so-called “crush” technique for FNAC of solid tumors which involves gently smearing the tissue between two opposing glass slides.
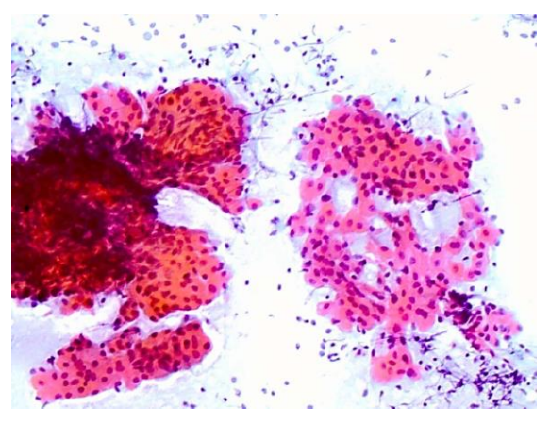
Figure 5: Example of a smear showing oncocytic cells and lymphocytes that is too thick on the left, and a desired thickness (monolayer) on the right
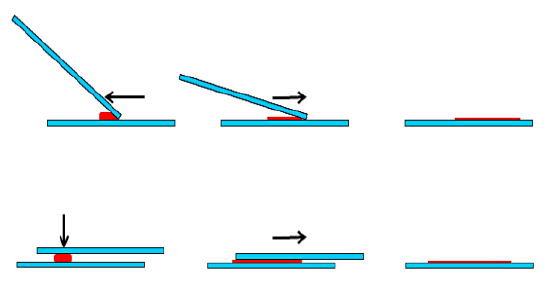
Figure 6: “Pull-push” (above) & “crush” (below) smear techniques
Fixing the smear
Smears are immediately fixed to avoid shrinkage artifact. This is achieved by either wet fixation or air-drying. Consult your cytopathologists about their preferred fixation method. As different stains are used for each technique, some prefer using both techniques for the same specimen, as the two methods can produce complementary results.
Wet-fixation technique
This is achieved by dipping the slide into 95% ethanol solution, or by applying a spray fixative (Figure 3). Spray fixatives typically consist of an admixture of polyethylene glycol and ethyl alcohol or isopropyl alcohol. The alcohol evaporates and leaves the glycol covering the smear. Material fixed by wet-fixation technique can be stained both with Papanicolaou (PAP) and hematoxylin-eosin (H&E) stains (Figure 7).
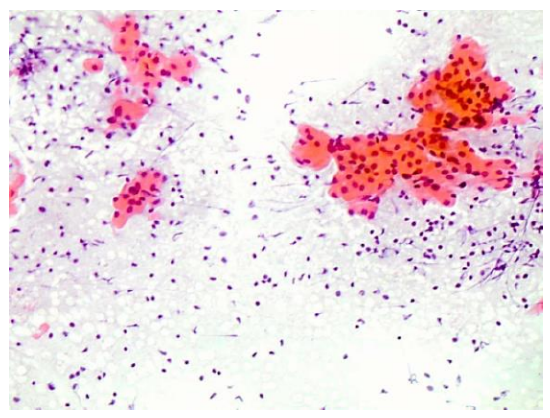
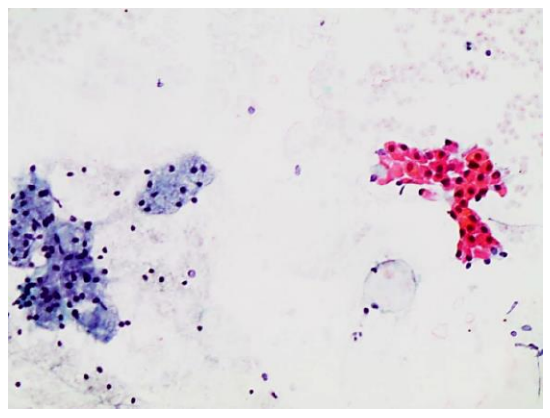
Figure 7: Examples of an H&E smears showing oncocytic cells and lymphocytes
Air-drying fixation technique
The smeared material is allowed to air-dry; drying should be quick and may be aided with a hairdryer or a fan. The WrightGiemsa stain is used for air-dried smears.
Labeling
Label the slides with the patient's details and the origin of the aspirate. The slides in Figure 8 have been sandblasted at one end so that details can be written on the slide with pencil.
Figure 8: Sandblasted end of slides for labeling with pencil
Liquid based cytology
This technique is useful for hypocellular aspirates. Instead of being spread onto a glass slide, the material obtained by aspiration or by cytobrushing is transferred to a vial of fixative. The vial is then centrifuged, and the sediment is used for the smear.
Exfoliative or brush cytology
Brush cytology is cheap, non-invasive, and virtually painless and requires minimal training. Although it is highly specific, it is less sensitive i.e. negative brush cytology does not rule out malignancy. It is therefore useful for screening suspicious oral lesions; if dysplastic cells or molecular alterations are identified, patients should be referred for tissue biopsy.
Figure 9 shows a close-up view of the tip of a cytobrush. A toothbrush is a reliable and cheap alternative (Figure 10).

Figure 9: Tip of cytobrush
Figure 10: Toothbrush is a reliable and cheap alternative for a cytobrush1
Method of brush cytology
Apply the cytobrush to the oral lesion with enough pressure to slightly bow the handle
- Rotate the cytobrush through 360° while applying pressure to the lesion
- Punctate bleeding indicates that the basement membrane has been breached and an adequate tissue depth has been sampled
- Deposit the brushings onto a microscope glass slide by rolling the brush on the surface of the slide through 360°
- Immediately fix the smear in accordance with the technique previously described with FNAC
- Label the slides with the patient's details and the origin of the aspirate
Trucut biopsy
Percutaneous core biopsy allows one to harvest a solid core of tumor for histological examination in an ambulatory setting. It is generally done with a Trucut biopsy system. Unlike FNAC, Trucut biopsy can seed tumor cells; therefore, avoid using it for salivary gland tumors, melanomas etc. unless the tumor is considered to be inoperable.
An outer cannula unsheathes a sharp-pointed needle that can be retracted and advanced into and out of the cannula. The needle has a notch which traps a core of tissue which has been sliced off the tumor by the sharp end of the outer cannula (Figures 11a-d). It requires a two-handed technique, although automated and semiautomated systems are also available.
Method
- Clean and infiltrate the skin with local anesthetic at the intended biopsy site
- Steady the mass with the non-dominant hand
- Make a small (3 mm) stab incision in the skin with a scalpel
- With the central needle retracted into the cannula (Figures 11 a, b), advance the Trucut needle and cannula into the mass taking care to avoid large vessels
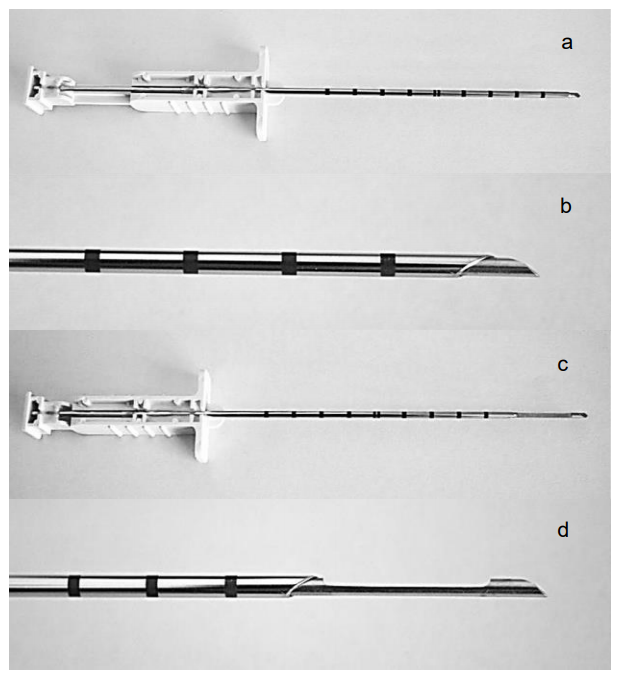
Figure 11: Trucut biopsy needle with plunger retracted (a, b) and plunger advanced (c, d)
- Steady the position of the handle of the outer cannula with the non-dominant hand
- Advance the plunger (which is attached to the needle) so that the needle extrudes from the tip of the cannula into the mass (Figures 11 c, d); this allows tumor tissue to fill the notch in the needle
- Steady the position of the handle of the needle with the dominant hand while rapidly advancing the handle of the outer cannula with the non-dominant hand so that the cannula is advanced over the notch of the needle, slicing off and trapping tumor tissue that has prolapsed into the notch
- Withdraw the Trucut apparatus from the patient
- Advance the handle of the needle to expose the core of tissue (Figure 12)
- Place the core of tissue in a specimen jar with formaldehyde
- Apply light pressure to the incision for hemostasis

Figure 12: Core of tissue in the notch of the Trucut needle
Biopsy of oral and oropharyngeal mucosal tumors
Mucosal tumors of the upper aerodigestive tract are typically biopsied under local anesthesia in the office or outpatient clinic. Topical anesthesia is applied to the mucosa following which local anesthesia (1% or 2% lidocaine with 1:100,000 epinephrine) is injected into the surrounding submucosa or a nerve block is done e.g. of the lingual nerve (http://emedicine. medscape.com/article/82850-overview).
Once well anesthetized, a punch biopsy is taken with Blakesley forceps, taking care not to crush the tissue. A through-cutting Blakesley forceps is preferred as it does not tear tissue and causes less crush artifact (Figure 13). Bleeding is generally minor and stops spontaneously or can be arrested with chemical cautery using a silver nitrate stick (Figure 14).
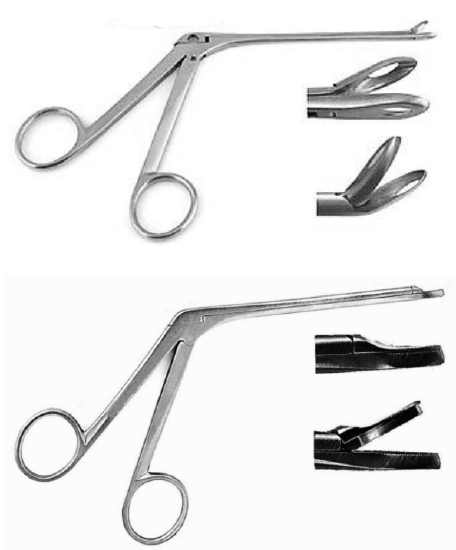
Figure 13: Blakesley nasal forceps (above); through-cutting Blakesley forceps (below)

Figure 14: Silver nitrate sticks used for hemostasis
Punch Biopsy of Skin
Punch biopsy is used for pigmented skin lesions, neoplasms, inflammatory and chronic disorders of skin. It is done under local anesthesia with a circular blade attached to a handle that is rotated as it is advanced to produce a 3-4 mm cylindrical full thickness core of skin (Figure 15).

Figure 15: 3mm/4mm punch
Method
- Determine the direction of Langer’s lines (lines of skin tension); stretching the skin perpendicular to the lines while doing the biopsy results in an elliptical-shaped wound with the long axis along Langer’s lines and allows for easier closure with a single suture and a better cosmetic result (Figure 16).
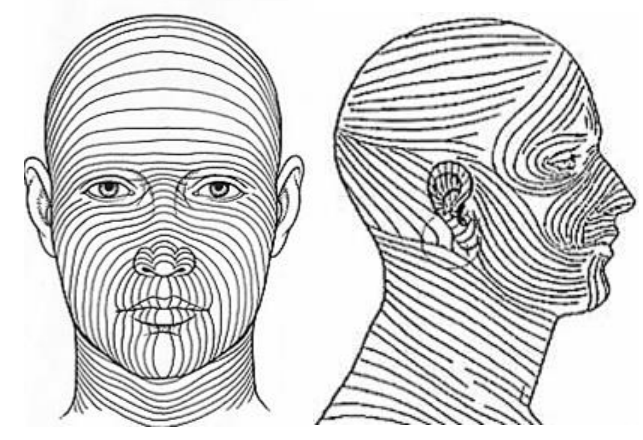
Figure 16: Langer’s lines / Tension lines
- Select an appropriate punch biopsy instrument (3 or 4 mm)
- Clean and infiltrate the skin with 2% lidocaine with epinephrine
- Stretch the skin at 90o to Langer’s lines between thumb and index finger of the non-dominant hand
- Apply the punch vertically to the skin and rotate the punch between thumb and index finger of the dominant hand, cutting through epidermis, dermis and into subcutaneous fat (Figures 17, 18)
- Remove the punch
- Gently elevate the 3-4 mm cylindrical core of skin with a needle to avoid crush artifact, and cut it free at its base with iris scissors
- Only larger punch biopsy sites require closure with a single nylon suture

Figure 17: Appropriate diameter punch applied to skin
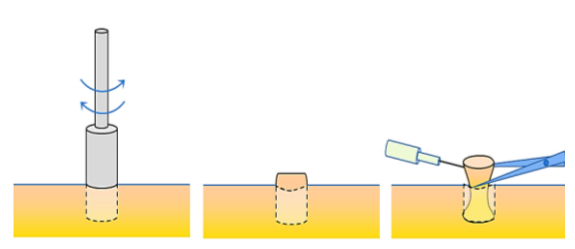
Figure 18: Rotate punch through skin, and divide the specimen with fine scissors below the dermis while holding it with a needle
Open Surgical Biopsy
Open surgical biopsy is indicated when FNAC and Trucut biopsy are inconclusive or are suggestive of lymphoma.
Anesthesia: Biopsy is done under local or general anesthesia. Because taking a biopsy of a deep-seated lymph node is often more difficult than expected, especially for a surgeon not well versed in the detailed surgical anatomy of the neck, one should have a low threshold for doing such biopsies under general anesthesia.
Hemostasis: At the conclusion of the surgery, ask the anesthetist to do a Valsalva procedure to identify venous bleeders. Have a low threshold for leaving a drain in place to prevent a hematoma.
Skin incisions: Incisions are made along Langer’s lines (Figure 16); when a neck dissection may subsequently be required, incisions should be made along neck dissection incision lines.
Lymph node biopsy: Lymph nodes should ideally be completely excised rather than incised to avoid contaminating the neck with cancer cells of an unsuspected cervical metastasis.
Submandibular triangle (Level 1) biopsy: Masses of the submandibular gland are removed by resecting the whole gland. Removing lymph nodes in the vicinity of the mandibular ramus puts the marginal mandibular nerve at risk of injury; it is safest and cosmetically best to place the incision in the vicinity of the hyoid bone; to raise a superiorly based flap over Level 1 as for a neck dissection or as for excision of the submandibular salivary gland (See Submandibular gland excision); and to identify the marginal mandibular nerve where it crosses the facial vessels deep to platysma before excising the node (Figure 19).
Posterior triangle (Level V) lymph node biopsy: The spinal accessory nerve is very superficial in the posterior triangle, often just deep to skin in a thin patient. Injury to the nerve is a common cause of litigation. It exits the sternocleidomastoid muscle to enter the posterior triangle about 1 cm behind Erb’s point which is at the junction of the upper and middle thirds of the muscle where the cervical plexus branches exit (Figure 20). Avoid muscle relaxants so that mechanical or electrical stimulation can be used to help identify the nerve
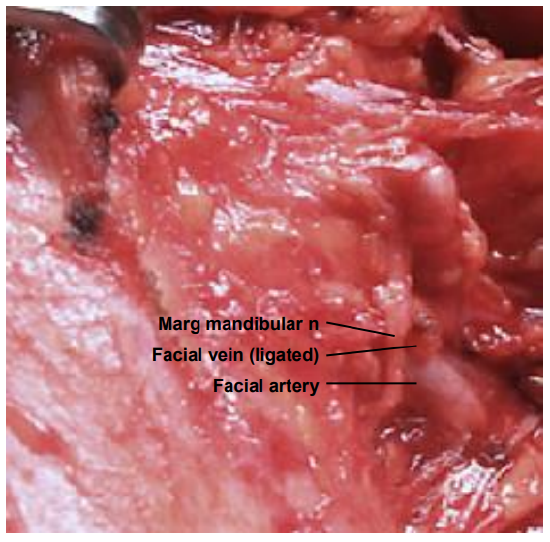
Figure 19: The marginal mandibular nerve is seen crossing the facial artery and vein (right neck)
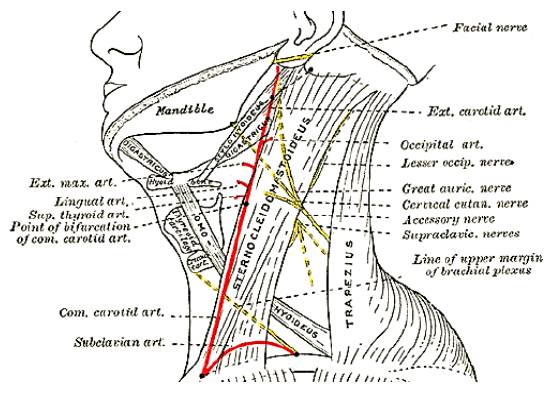
Figure 20: Course of spinal accessory nerve (Wikipedia)
Parotid biopsy: The principal concern is injury to the facial nerve. Other than in the hands of an experienced parotid surgeon, a mass should not simply be excised, but removed by formal partial parotidectomy with identification of the facial nerve (See Parotidectomy).
Nasal & Nasopharyngeal tumors
Tumors in the nasal cavity and nasopharynx can generally be biopsied in the office/outpatients department under local anesthesia with the exception of vascular tumors such as angiofibromas. Angiofibromas should be suspected in younger males and should not be biopsied.
Ambulatory endoscopic biopsy technique of the nasopharynx
- Patient inhales topical local anesthetic (4% lidocaine) and decongestant (1% phenylephrine) through the nose to ease the passage of instruments through the nose and because biopsy of normal nasopharyngeal mucosa causes discomfort
- Inspect both nasal cavities and the nasopharynx with a flexible or rigid endoscope (Figure 21)
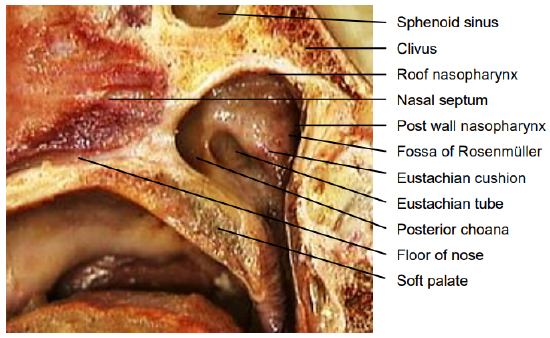
Figure 21: Sagittal view of nasopharynx
- Examine the mass and note the easiest transnasal approach for the biopsy forceps
- Gently pass a Blakesley nasal forceps blindly along the floor of the nose until it stops against the nasopharynx
- Tilt the patient’s head back by about 30o so that the Blakesley forceps does not drop out of the nose when released
- Pass a flexible or rigid endoscope up the contralateral (or ipsilateral) nasal cavity
- Visualize the tip of the biopsy forceps in the nasopharynx
- Biopsy the tumor under direct vision and withdrew the forceps and specimen
- Significant bleeding is unusual
Transoral biopsy technique of the nasopharynx under general anesthesia
- Intubate the patient orally
- Place the patient in a tonsillectomy position
- Insert a tonsillectomy (Boyle Davis) gag
- Pass a suction catheter through each nasal cavity, around the soft palate, and out the mouth and retract the palate anteriorly
- The nasopharynx can then be viewed using a warmed laryngoscopy mirror placed in the oropharynx either directly with a headlight or through an operating microscope (Figure 22)
- Biopsies can then be taken either transorally or transnasally under vision
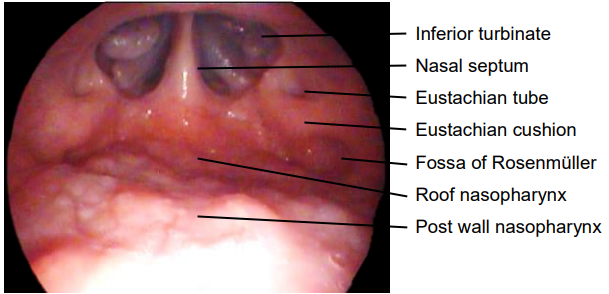
Figure 22: Transoral view of nasopharynx with laryngoscopy mirror
- Alternatively, one can displace the soft palate anteriorly with a Yankauer postnasal speculum (Figure 23) and take the biopsy through the speculum under direct vision
Pitfalls of nasopharyngeal biopsy
- Avoid transnasal biopsy without direct endoscopic visualization of the mass
- Bleeding diathesis
- Vascular masses
- Lesions near the carotid artery i.e. of the lateral wall behind fossa of Rosenmuller (Figures 21, 22)
- Ectatic internal carotid arteryInadequate local anesthesia
- Cystic lesion causing CSF leak
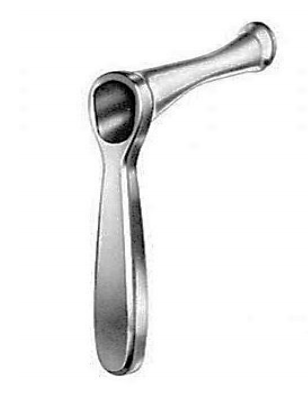
Figure 23: Yankauer postnasal speculum
References
- Babshet M et al. Efficacy of oral brush cytology in the evaluation of the oral premalignant and malignant lesions. J Cytol 2011;28(4):165-72
Authors
Kathy Taylor MBChB, DCH (SA), MMed
(Anat Path)
Anatomical pathologist
Pathcare
Cape Town, South Africa
kathy.taylor@pathcare.co.za
Ellen Bolding MBChB, DCH (SA), MMed
(Anat Path)
Anatomical pathologist
Pathcare
Cape Town, South Africa
boldred@pathcare.co.za
Author & Editor
Johan Fagan MBChB, FCS(ORL), MMed
Professor and Chairman
Division of Otolaryngology
University of Cape Town
Cape Town, South Africa
johannes.fagan@uct.ac.za

The Open Access Atlas of Otolaryngology, Head & Neck Operative Surgery by Johan Fagan (Editor) johannes.fagan@uct.ac.za is licensed under a Creative Commons Attribution - Non-Commercial 3.0 Unported License



