12.8: Zinc
- Page ID
- 41000
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Many animal products are good sources of zinc and are estimated to account for 70% of the zinc North Americans’ consume1. An estimated 15-40% of consumed zinc is absorbed2. Zinc is taken up into the enterocyte through the Zir-and Irt-like protein 4 (ZIP4). Once inside the enterocyte, zinc can:
- Bind to the zinc storage protein thionein. Once thionein has bound a mineral (or a metal) it is known as metallothionein.
- Be used for functional purposes.
- Bind to the cysteine-rich intestinal protein (CRIP) where it is shuttled to a zinc transporter (ZnT). After moving through the basolateral membrane, zinc primarily binds to the circulating protein albumin3.
These functions are represented in the figure below.

Query \(\PageIndex{1}\)
The zinc attached to albumin is transported to the liver through the portal vein. There is not a major storage site of zinc, but there are pools of zinc in the liver, bone, pancreas, and kidney1. Zinc is primarily excreted in feces.
There are some similarities in how zinc and iron absorption are regulated. Increased zinc consumption results in increased thionein synthesis in the enterocyte. As a result, more zinc is bound to thionein (forming metallothionein) and not used for functional uses or transported into circulation, as represented by the thick and thin arrows in the figure below.

The enterocytes are then sloughed off preventing the bound zinc from being absorbed.

There are a 3 inhibitors of zinc absorption (same inhibitors you learned about of iron absorption)3:
Phytate (phytic acid)
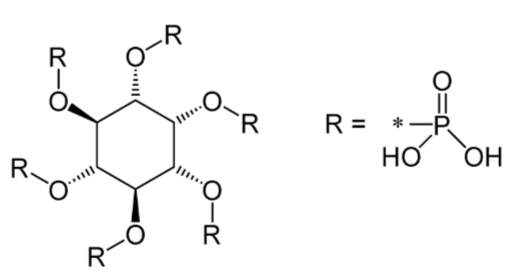
Polyphenols (coffee, tea)3

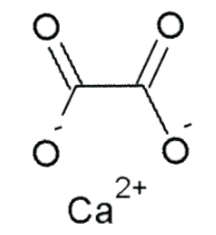
Oxalate (spinach, rhubarb, sweet potatoes, and dried beans)3
Non-heme iron also inhibits zinc absorption.
Query \(\PageIndex{2}\)
In supplements, zinc is found as3,7:
- Zinc oxide - 80% zinc
- Zinc chloride - 23% zinc
- Zinc sulfate - 23% zinc
- Zinc gluconate - 14.3% zinc
Zinc oxide is the least bioavailable form, but since it is 80% zinc, it is commonly used in supplements7.
Query \(\PageIndex{3}\)
Zinc Functions
Zinc is a cofactor for up to 300 enzymes in the body1. Enzymes that use zinc as a cofactor are known as metalloenzymes.
Zinc is a cofactor for the antioxidant enzyme superoxide dismutase that converts superoxide to hydrogen peroxide, as shown below.

Alcohol dehydrogenase uses 4 zincs per enzyme. Its role in ethanol metabolism is shown below3.

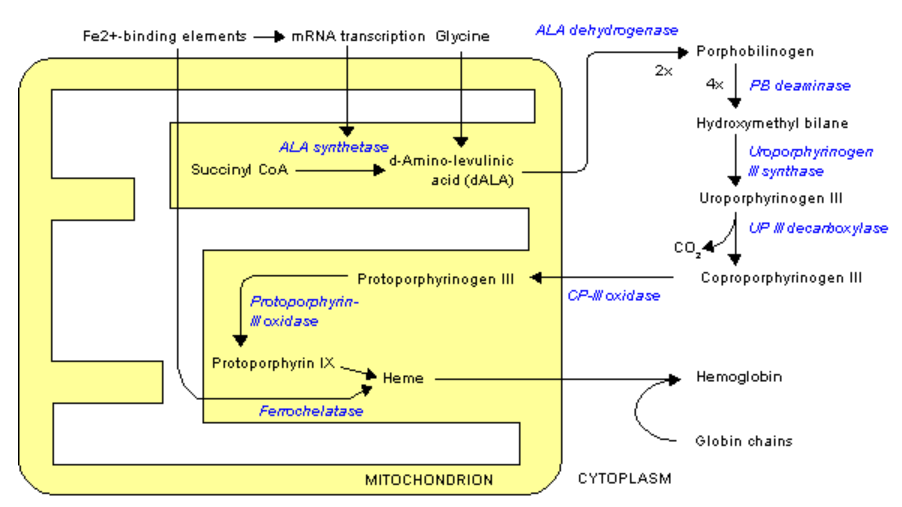
Delta-aminolevulinic acid dehydratase (ALA dehydrogenase), which is involved in heme synthesis, uses 8 zincs/enzyme to form porphobilinogen, as shown below3.
The enzyme that cleaves the extra glutamates from folate so that it can be taken up into the enterocyte is a metalloenzyme3. The cleavage of folate is shown in the figure below.

Other notable metalloenzymes include DNA and RNA polymerase3.
Zinc is also important for the formation of zinc fingers in proteins. Zinc fingers help proteins bind to DNA3.

Zinc is also important for growth, immune function, and reproduction9.
A Cochrane systematic review concluded that when taken within 24 hours of the onset of symptoms, that zinc lozenges or syrup results in a significant decrease in the duration and severity of common cold symptoms12. Thus, commonly used zinc lozenges may be an effective way to combat the common cold. However, large amounts of zinc consumption can be problematic for copper and ultimately iron levels in the body, as described in the copper section.
Query \(\PageIndex{4}\)
Zinc Deficiency & Toxicity
As can be seen on the bottom map in the link below, the risk of zinc deficiency is low in North America, but there are other places in the world where it is much more common.

At particular risk are children, pregnant women, elderly and the poor13. Symptoms of zinc deficiency include1,3:
- Growth inhibition
- Delayed sexual maturation
- Dermatitis
- Hair loss
- Impaired immune function
- Skeletal abnormalities
In the link below you can see a picture of an infant with dermatitis caused by zinc deficiency.
Web Link
Another cause of zinc deficiency is mutation of ZIP4 that results in the condition acrodermatitis enteropathica. Without ZIP4, zinc cannot be taken up efficiently into the enterocyte. This condition is managed by administering very high levels of zinc, some of which is absorbed through other mechanisms3.
Zinc toxicity is not common, but an acute toxicity results in1:
- Nausea
- Vomiting
- Intestinal cramps
- Diarrhea
Chronic toxicity can result in copper deficiency, as will be discussed in the copper section3.
Query \(\PageIndex{5}\)
References
- Byrd-Bredbenner C, Moe G, Beshgetoor D, Berning J. (2009) Wardlaw's perspectives in nutrition. New York, NY: McGraw-Hill.
- Whitney E, Rolfes SR. (2011) Understanding nutrition. Belmont, CA: Wadsworth Cengage Learning.
- Gropper SS, Smith JL, Groff JL. (2008) Advanced nutrition and human metabolism. Belmont, CA: Wadsworth Publishing.
- en.Wikipedia.org/wiki/File:Phytic_acid.png
- en.Wikipedia.org/wiki/File:Gallic_acid.svg
- en.Wikipedia.org/wiki/File:Calcium_oxalate.png
- Bowman BA, Russell RM, editors. (2006) Present knowledge in nutrition. Washington, DC: International Life Sciences Institute Press.
- https://s3-us-west-2.amazonaws.com/c...BC92E7E74A.png
- https://courses.lumenlearning.com/suny-nutrition/chapter/6-5-alcohol-metabolism/
- en.Wikipedia.org/wiki/File:Heme_synthesis.png
- https://s3-us-west-2.amazonaws.com/c...555905FFA8.png
- Singh M, Das RR. (2011) Zinc for the common cold (Review). The Cochrane Collaboration.
- Wessells KR, Brown KH. (2012) Estimating the Global Prevalence of Zinc Deficiency: Results Based on Zinc Availability in National Food Supplies and the Prevalence of Stunting. PLoS ONE 7(11): e50568


