17.1: Metabolism Basics
- Page ID
- 36325
- Describe examples of catabolic and anabolic metabolism.
Introduction
You learned in previous chapters about macronutrients, their digestion and absorption. In this chapter, we are taking a closer look 'what the body does with the absorbed nutrients' to promote health and wellbeing; mainly food is converted into cellular energy (as ATP) and occurs in three stages. This energy is used by the body to move muscles (mechanical energy), to generate heat to warm you, to conduct nerve impulses in form of electrical energy, and to produce energy stores in form of glycogen and fat.
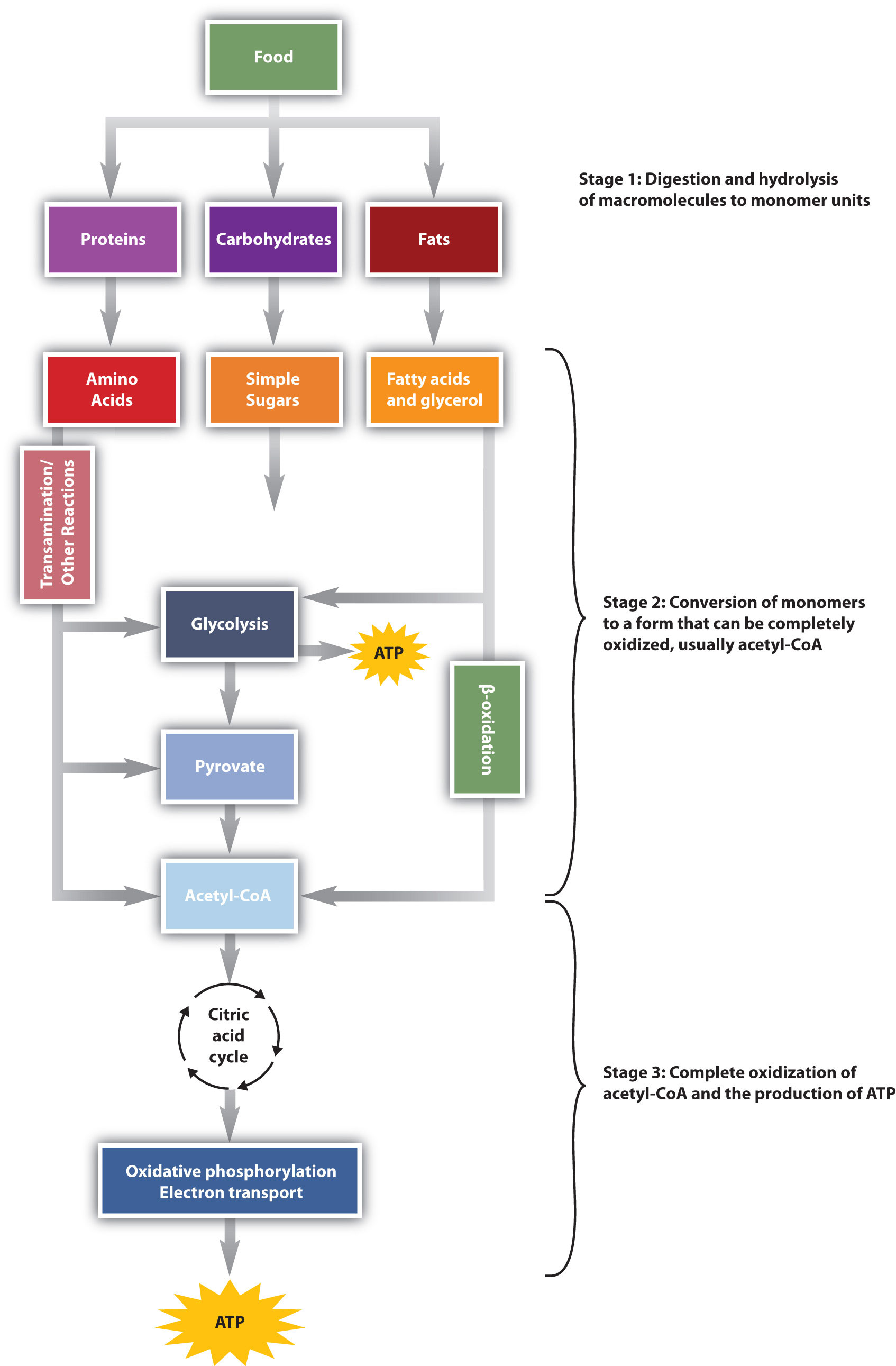
Figure 9.1 Energy Conversions.1 (Please note that 'pyruvate' is a metabolite that is generated during glycolysis; hence, please ignore the typo in the figure above)
At all times, your cells produce a steady supply of energy - either from the foods you eat or from energy stores inside the body; understanding these intricate reactions referred to as 'metabolism', is an important aspect of studying nutrition.
Definition of metabolism
Metabolism consists of all the (bio)chemical processes that occur in living cells. These processes (a.k.a. biochemical reactions) can generally be classified as either anabolic or catabolic. Anabolic means to build, catabolic means to breakdown. If you have trouble remembering the difference between the two, recall that anabolic steroids are what is used to build enormous muscle mass. Metabolism allows the body to convert energy from foods into energy needed for physical activity or building life-sustaining substances.

Figure 9.2 One of these two is taking anabolic steroids, which one would be your guess?2
Introduction to anabolic and catabolic reactions
An anabolic pathway requires energy to build something. For example, the body uses a single amino acid to form proteins, excess glucose molecules are combined to make glycogen, and excess fatty acids attach to glycerol molecules to make triglycerides; these reactions involve the formation of new bonds, which requires energy. On the other hand, a catabolic pathway generates energy by breaking down something. This is shown in the example below of glucose and glycogen. The same is true for other macronutrients, such as lipid breakdown (or catabolism) into fatty acids and proteins breakdown (or catabolism) into their amino acid components.

Figure 9.3 The breakdown of glycogen to glucose is catabolic. The glucose can then be used to produce energy. The synthesis of glycogen from glucose is anabolic and requires energy.3
Anabolic and catabolic can be used to describe most metabolic processes in the body. For instance, after a meal, there is often a positive energy balance, which means that there might be more energy and macronutrients available than the body needs at that time. Thus, some energy is stored and nutrients are used for synthesis, such as amino acids being used for protein or lipid synthesis. However, after a fast, or a prolonged period without energy intake, the body may go into negative energy balance which is considered catabolic. In this 'negative energy' condition, macronutrients will be mobilized from their stores to be used to generate energy. For example, if prolonged enough, muscle protein can be broken down, and some of the released amino acids can be converted into glucose to be used as an energy source.
Metabolism Takes Place within Cells
The chemical reactions involved in energy production and storage take place within the body’s cells. Energy production takes place in mitochondria, whereas ribosomes help to manufacture proteins, and the smooth endoplasmic reticulum produces lipids that can be used by other organelles.1 Mitochondria are considered the 'powerhouse of each cell' as they generate most of the cellular energy through aerobic metabolism. Almost all body cells contain mitochondria (with the exception is the red blood cells, which produce energy anaerobically in the cytoplasm).4

Figure 9.4. Catabolic and Anabolic Reactions. Catabolic reactions involve the breakdown of molecules into smaller components, whereas anabolic reactions build larger molecules from smaller molecules. Catabolic reactions usually release energy whereas anabolic processes usually require energy.4
The Liver Plays a Central Role in Metabolism
The most metabolically active organ in the body is the liver. In previous modules you learned that carbohydrates, fats and proteins found in in foods are digested and absorbed as monosaccharides, glycerol and fatty acids, as well as amino acids respectively. Once these nutrients have been absorbed, the liver is the first organ to metabolize them in order to send them to other tissues via the bloodstream. The liver also uses these nutrients to synthesize new compounds—for instance, converting essential amino acids into nonessential amino acids. The liver also stores some of these nutrients—glycogen, for example—to use in the future.4
Metabolism is based on (bio)chemical processes, including oxidation and reduction
A number of metabolic reactions oxidize or reduce compounds. A compound that is being oxidized loses at least one electron, while a compound that is reduced gains at least 1 electron. To remember the difference, a mnemonic device such as OIL (oxidation is lost), RIG (reduction is gained) is helpful. Oxidation-reduction reactions are illustrated in the figure below.

Figure 9.5 The purple compound is being oxidized, the orange compound is being reduced1
Another way to remember oxidation versus reduction is LEO goes GER (like a lion)
Lose Elections = Oxidize
Gain Elections = Reduce
Iron is a good example we can use to illustrate oxidation-reduction reactions. Iron commonly exists in two oxidation states (Fe3+ or Fe2+). It is constantly oxidized/reduced back and forth between the two states. The oxidation/reduction of iron is shown below.
Fe3+ + e- → Fe2+ Reduced
Fe2+ → Fe3+ + e- Oxidized
However, some oxidation-reduction reactions are not as easy to recognize. There are some simple rules to help you recognize less obvious oxidation/reduction reactions that are based upon the gain or loss of oxygen or hydrogen. These are as follows:
Oxidation: gains oxygen or loses hydrogen
Reduction: loses oxygen or gains hydrogen
Metabolism: aerobic vs. anaerobic pathways to generate energy
The human body is able to generate energy during times of adequate and inadequate oxygen supply. The metabolic steps to generate energy in form of 'ATP' differ in aerobic and anaerobic pathways (which will be covered in more detail later in the segment).
Important to note right now is the following:
- many catabolic reactions require adequate oxygen supply and prefer the aerobic pathways
- in times of oxygen shortage, let's say you are running a 100-yard sprint, your body can provide energy to your muscles through anaerobic pathways
The following Crash Course video provides an excellent introduction to Nutrition & Metabolism:
References & Attributions
- Blake, J. S., Munoz, K. D., & Volpe, S. (2019). Nutrition: From Science to You (4th ed.). Pearson. [Not sure how I feel about referencing one nutrition book in another... NH]
Images
- https://med.libretexts.org/Courses/Skyline_College/Chemistry_for_Health_Sciences/Book%3A_The_Basics_of_GOB_Chemistry_(Ball_et_al.)/19%3A_Energy_Metabolism/19.03%3A_Stage_I_of_Catabolism
- http://en.Wikipedia.org/wiki/Image:G...e_Platform.jpg
- https://open.oregonstate.education/humannutrition/chapter/chapter-2-energy-yielding-macronutrients/
- https://wou.edu/chemistry/files/2018...d-anabolic.png (alternative: CC https://2012books.lardbucket.org/boo...-overview.html)
Content on this page is adapted from the following sources under an open-licensing agreement:
Additional contributions made by Claudia Kelley as part of the ASCCC 2020 OERI.

