2.7: Lipids - Triglycerides, Phospholipids and Sterols
- Page ID
- 41717
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Triglycerides
Triglycerides are the most common lipid in our bodies and in the foods we consume. Fatty acids are not typically found free in nature, instead they are found in triglycerides. Breaking down the name triglyceride tells a lot about their structure. "Tri" refers to the three fatty acids, "glyceride" refers to the glycerol backbone that the three fatty acids are bonded to. Thus, a monoglyceride contains one fatty acid, a diglyceride contains two fatty acids. Triglycerides perform the following functions in our bodies:
- Provide energy
- Primary form of energy storage in the body
- Insulate and protect
- Aid in the absorption and transport of fat-soluble vitamins.
A triglyceride is formed by three fatty acids being bonded to glycerol as shown below.
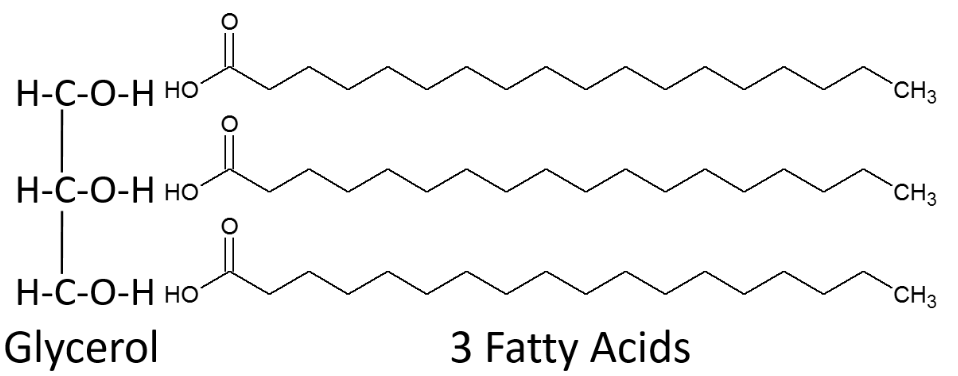
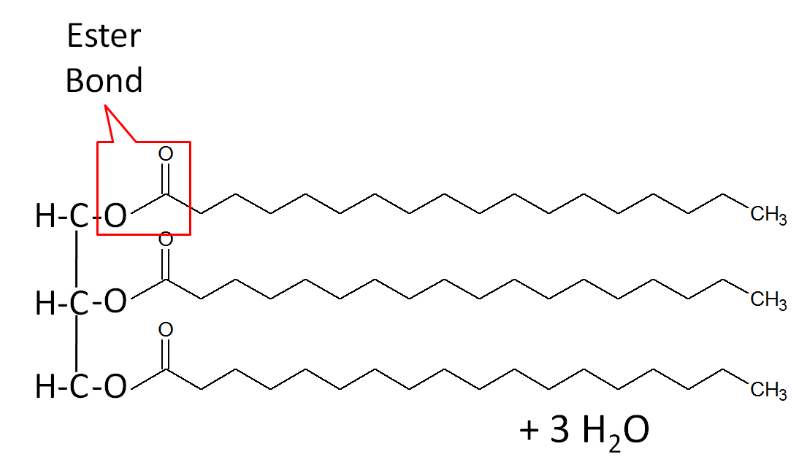
When a fatty acid is added to the glycerol backbone, this process is called esterification. This process is so named because it forms an ester bond between each fatty acid and glycerol. Three molecules of water are also formed during this process as shown below.
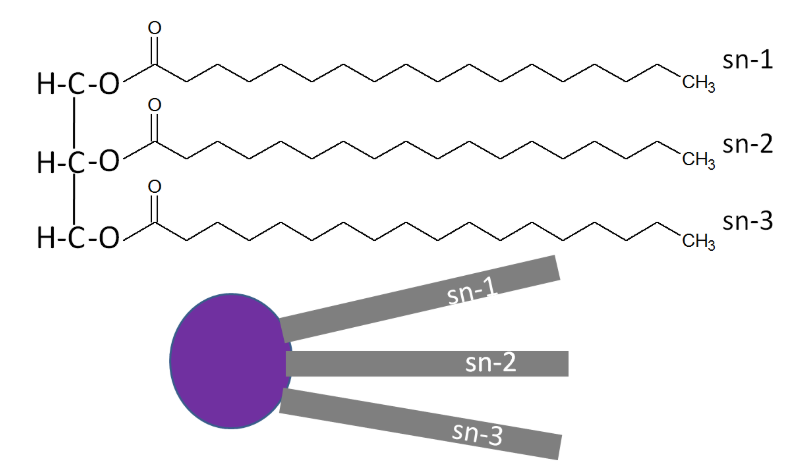
A stereospecific numbering (sn) system is used to number the three fatty acids in a triglyceride sn-1, sn-2, and sn-3 respectively. A triglyceride can also be simply represented as a polar (hydrophilic) head, with 3 nonpolar (hydrophobic) tails, as shown below.
The three fatty acids in a triglyceride can be the same or can each be a different fatty acid. A triglyceride containing different fatty acids is known as a mixed triglyceride. An example of a mixed triglyceride is shown below.

Phospholipids
Phospholipids are similar in structure to triglycerides, with the only difference being a phosphate group and a nitrogen-containing compound in the place of a fatty acid. The best known phospholipid is phosphatidylcholine (aka lecithin). As you can see in the structure below, it contains a choline off of the phosphate group. It can have a number of different fatty acids in its structure (fatty acids differ from tissue-to-tissue and between species).

However, you will not normally find phospholipids arranged like a triglyceride, with the 3 tails opposite of the glycerol head. This is because the phosphate/nitrogen group of the phospholipid is hydrophilic. Thus, the structure will look like the 2 figures below.


Similar to triglycerides, phospholipids are also represented as a hydrophilic head with two hydrophobic tails as shown below.

Phospholipid Functions
Because its structure allows it to be at the interface of water-lipid environments, there are two main functions of phospholipids:
- Key Component of Cells' Lipid Bilayers
- Emulsification
Number 1 in the figure below is a cell's lipid bilayer, while 2 is a micelle that is formed by phospholipids to assist in emulsification.

1. Key Component of Cells' Lipid Bilayers
Phospholipids are an important component of the lipid bilayers of cells. A cross section of a lipid bilayer is shown below. The hydrophilic heads are on the outside and inside of the cell; the hydrophobic tails are in the interior of the cell membrane.

2. Emulsification
As emulsifiers, phospholipids help hydrophobic substances mix in a watery environment because of their amphipathic (has hydrophobic and hydrophilic) properties. It does this by forming a micelle as shown below. The hydrophobic (water fearing) substance is trapped on the interior of the micelle away from the aqueous environment.

As a result, it can take a hydrophobic liquid (oil) and allow it to mix with hydrophilic (water loving) liquid (water).
Foods rich in phosphatidylcholine include: egg yolks, liver, soybeans, wheat germ, and peanuts7. Egg yolks serve as an emulsifier in a variety of recipes. Your body makes all the phospholipids that it needs, so they do not need to be consumed (not essential).
Sterols
The last category of lipids are the sterols. Their structure is quite different from the other lipids because sterols are made up of a number of carbon rings. The generic structure of a sterol is shown below.
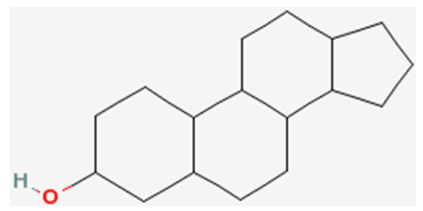
The primary sterol that we consume is cholesterol. The structure of cholesterol is shown below.
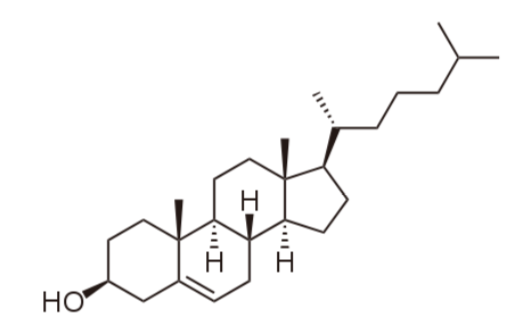
Cholesterol is frequently found in foods as a cholesterol ester, meaning that there is a fatty acid attached to it. The structure of a cholesterol ester is shown below.
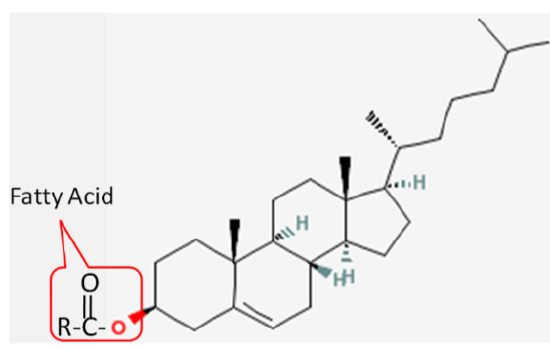
All sterols have a similar structure to cholesterol. Cholesterol is only found in foods of animal origin. If consumers were more knowledgeable, intentionally misleading practices, such as labeling a banana “cholesterol free”, would not be as widespread as they currently are today.
Function
Although cholesterol has acquired the status of a nutrition "villain", it is a vital component of cell membranes and is used to produce vitamin D, hormones, and bile acids. You can see the similarity between the structures of vitamin D and estradiol, one of the forms of estrogen shown below.

We do not need to consume any cholesterol from our diets (not essential) because our bodies have the ability to synthesize the required amounts. The figure below gives you an idea of the cholesterol content of a variety of foods.
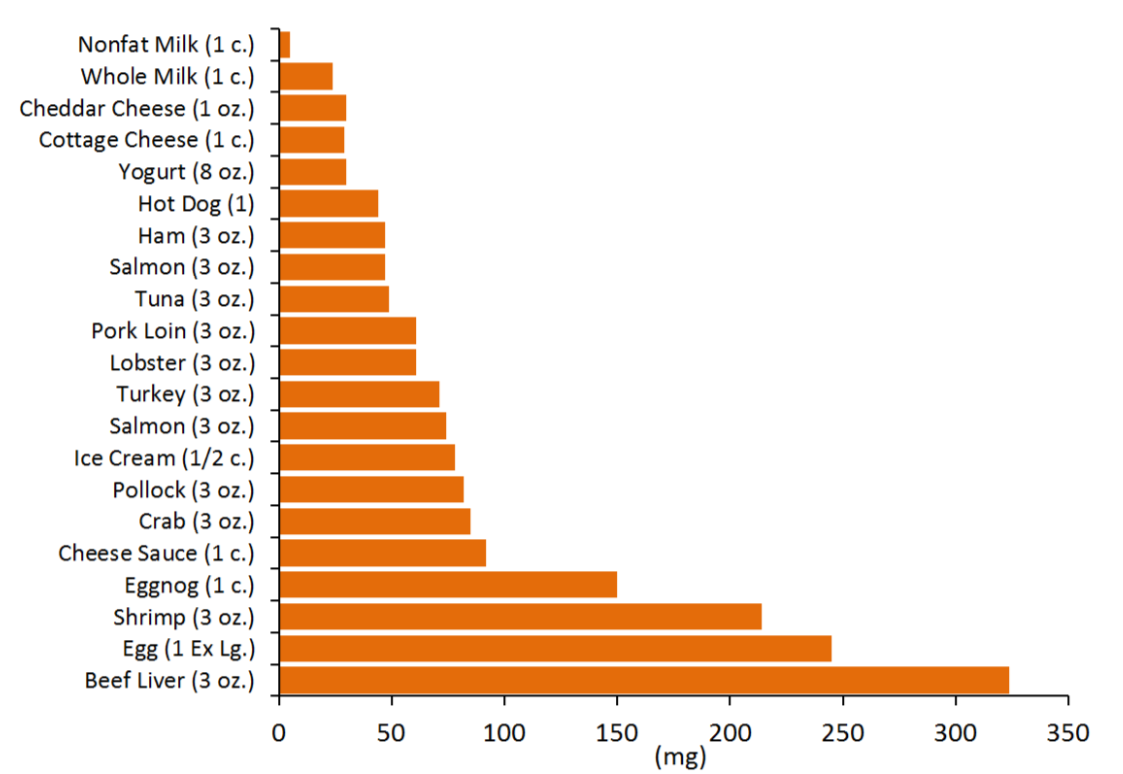
There is neither bad nor good cholesterol, despite these descriptions being commonly used for low-density lipoprotein and high-density lipoprotein, respectively. Cholesterol is cholesterol. High-density lipoprotein and low-density lipoprotein contain cholesterol but are actually lipoproteins that are described in chapter 4.
References
- commons.wikimedia.org/wiki/Fi...pc_details.svg
- en.Wikipedia.org/wiki/File:Ph...dylcholine.png
- en.Wikipedia.org/wiki/File:Li...nd_micelle.png
- en.Wikipedia.org/wiki/File:Bi...on_profile.svg
- en.Wikipedia.org/wiki/Micell..._scheme-en.svg
- en.Wikipedia.org/wiki/File:Emulsions.svg
- Byrd-Bredbenner C, Moe G, Beshgetoor D, Berning J. (2009) Wardlaw's perspectives in nutrition. New York, NY: McGraw-Hill.
- en.Wikipedia.org/wiki/File:Cholesterol.svg
- en.Wikipedia.org/wiki/File:Cholecalciferol.svg
- en.Wikipedia.org/wiki/File:Estradiol2.png
- http://ndb.nal.usda.gov/


